- Science Toys
- Magnetism
- Electromagnetism
- Electrochemistry
- Radio
- Thermodynamics
- Aerodynamics
- Light and optics
- Simple laser communicator
- Make your own 3D pictures
- Making permanent rainbows.
- A solar powered marshmallow roaster
- Make a spectroscope from a CD.
- The impossible kaleidoscope
- Make a solar hotdog cooker
- Exploring invisible light
- A high resolution spectrograph.
- Time-lapse photography.
- High speed photography.
- Stacking photos for high depth of field.
- Biology
- Mathematics
- Computers and Electronics
Exploring invisible light
At either end of the rainbow there is light we can't see. Below the red end is near infrared light, shorter in wavelength than the infrared we feel as heat. Above the violet end of the rainbow is near ultraviolet, longer in wavelength than the ultraviolet light that causes sunburn. Because these invisible lights are so close to the visible light we can see, they act in many ways just like normal light. They can be focused with lenses, and cameras can see them.
Fluorescent light
Seeing in the infrared
A source of near infrared light is as close as your television remote control.
Infrared remote control Look directly into the front of the remote control and push some buttons. You can't see anything change. But now point the remote at an inexpensive digital camera or video camera and push the buttons. Now, in the camera's screen, you see a brightly flashing beacon of light. You can use it as a flashlight to read by, as long as you look in the camera screen.

Infrared remote control Digital camera sensors can easily see in the infrared. In fact, they are more sensitive to infrared than they are to normal light. Camera manufacturers try to fix this by adding infrared blocking filters in front of the sensor. In cheap cameras, these are not very effective. In more expensive cameras, such as the digital single-lens reflex camera used to take the shot below, the infrared light is much dimmer than in the pocket camera shots above.

Infrared remote control
Seeing in the ultra-violet
Cheap ultraviolet light sources use a mercury vapor lamp and some dark purple glass that blocks most of the visible light from shining through. Unfortunately, rather a lot of blue and violet light gets through. A better source is an ultraviolet light emitting diode. While some of the cheap LEDs called "ultraviolet" are actually really just purple LEDs, you can obtain good LEDs whose wavelength is 395 nanometers or below. At the time of this writing, 395 nm LEDs can be had for a few dollars, while a 375 nm LED penlight goes for $25, and a 255 nm LED will set you back $600.00. It is not advisable to look into an ultraviolet LED, any more than it is to look at the sun. But a quick glance shows that a 395 nm LED looks dimly blue-violet to the human eye. But when we look at it through the camera, we can see that the camera sensor is again rather good at seeing this otherwise mostly invisible light. The light looks blue-white to the camera because the camera and its software interpret ultraviolet as blue, and this light is very bright in the ultraviolet.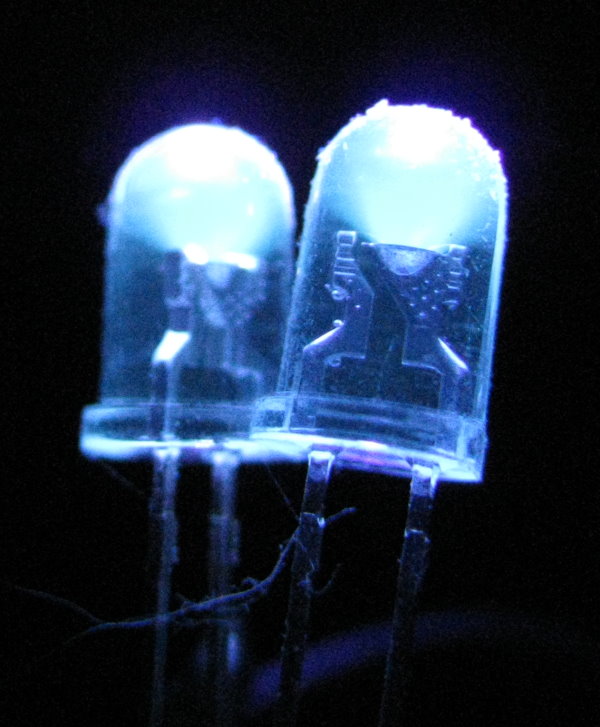
Ultraviolet light emitting diodes Bees can see in the ultraviolet. Flowers take advantage of this, and have ultraviolet pigments that bees can see but humans cannot. But when we take such a flower, in this case a California Poppy, and illuminate it with our 395 nanometer ultraviolet LED, and take a picture, we can see some of these markings. In the photo below, I took the picture on the left in normal light, and the picture on the right using the UV LED. You can see that the edges of the flower are lighter in color, and the center is darker, except for the stamens, which are very bright.

Poppy in visible and ultraviolet
Invisible ink
Invisible ink has many uses beyond secret communications between spies or criminals. One such use is as an anti-counterfeiting measure in money, as seen by the photo below, where a U.S. twenty dollar bill is lit from behind by one of our ultraviolet LEDs.
Twenty dollar bill in ultraviolet light In the bright green band of fluorescent ink, you can read the words "USA TWENTY".
How to see invisible ink
What we will need:- Invisible ink (either in a vial or in an invisible ink pen).
- A source of ultraviolet light, such as a UV light emitting diode.
- (Optional) Blue-blocking sunglasses (yellow goggles).

Ultraviolet LED with two CR2032 batteries Aim the light at the invisible writing. You may want to do this in a dark place for better readability. The blue-blocking sunglasses are useful for enhancing the contrast. They block most of the blue and violet light that leaks through the purple glass filters of mercury vapor lamp ultraviolet lights. They are still useful with the ultraviolet LEDs, even though those emit much less visible light.
How to make your own fluorescent dye
You can make your own fluorescent dye easily, after a trip to the kitchen for some powdered turmeric spice, and the bathroom for some rubbing alcohol. Put a teaspoon of turmeric into a glass, and add a few tablespoons of alcohol. Fold a paper towel into quarters to make a funnel, and filter the liquid into another glass. The photo below shows the liquid in a tiny glass vial, lit by our UV LEDs. You can use a cotton swab to write things with your new ink. It will appear yellow on the paper, and bright yellow-green when lit with ultraviolet light.
Tiny vial of turmeric dye
How does it do that?
A typical fluorescent dye (several are shown below) has many conjugated bonds, where double bonds and single bonds alternate in the molecule.
Curcumin molecule What is actually going on in the molecule is more complicated than the simple diagrams suggest, because the electrons that form those bonds aren't pinned down in one place, but tend to wander or smear out along the whole molecule. The electrons have many possible energy states. The lowest energy state (the ground state) is one where the electrons are closest to the nucleus of the atom, and are paired up, with each electron in the pair spinning in the opposite direction. We say that one electron is spin up, and the other is spin down.

Ethidium bromide molecule At room temperature, the electrons have enough heat energy to bounce around and bump one another between energy levels at random. But most of the electrons will be in the lower energy levels at any given time.

Fluorescein molecule The electrons in the molecule have energy levels of different types. The basic energy levels are the electronic states (the ground state, the excited ground states, and the exited triplet state). But layered on top of these are vibrational and rotational energy states.

Propidium iodide molecule These extra vibrational states allow the electron to absorb photons of different energies that raise the energy of the electron to different levels. Since the energy of a photon is related to its wavelength (the color of the light), many slightly different colors of light can be absorbed by the electron to push it into higher energy states.

Sulforhodamine B molecule Once an electron has absorbed a photon and has jumped into a higher energy state, it can lose some of the energy easily by releasing the vibrational energy as heat. The electron then settles into the lowest vibrational energy level at that excited electonic state. The three electronic states are diagrammed below. In the ground state, electrons are paired, one with spin up, and one with spin down. In the excited state, one of the electrons has a higher energy, but still has the opposite spin. In the third (triplet) state, one of the electrons has flipped its spin, so both electrons have the same spin.
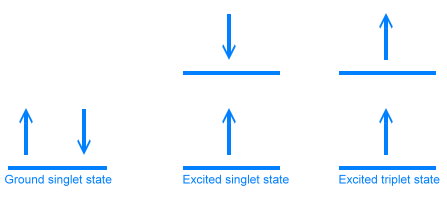
Electron states All of these energy levels and states come together in what is called the Jablonski diagram, which is used to show what is happening during fluorescence.
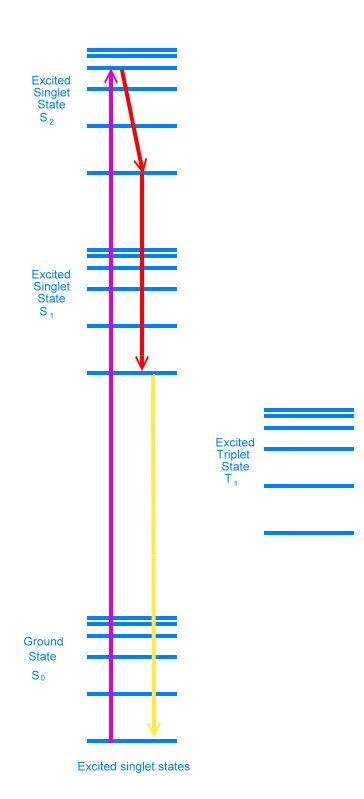
Jablonski diagram for fluorescence The diagram shows an electron in the ground state absorbing a photon and rising to the second excited singlet state (S2). This transition is shown in purple. Because the photon had more energy than was necessary to achieve the second electronic excited state, the electron is sitting at one of the vibrational energy levels in that electronic state. As time goes by, the electron loses energy as heat and settles into the bottom vibrational level of the second excited singlet state. It can lose more energy as heat and move down to the bottom of the excited singlet state 1, either in one step or in many small steps, stopping at various vibrational energy levels. These energy drops are shown in red in the diagram. It is possible for the electron to lose energy further, either by emitting a photon of light, or by what are called non-radiative means, where the energy ends up as heat. When a photon is emitted, we say the molecule has fluoresced. This is shown in yellow in the diagram.
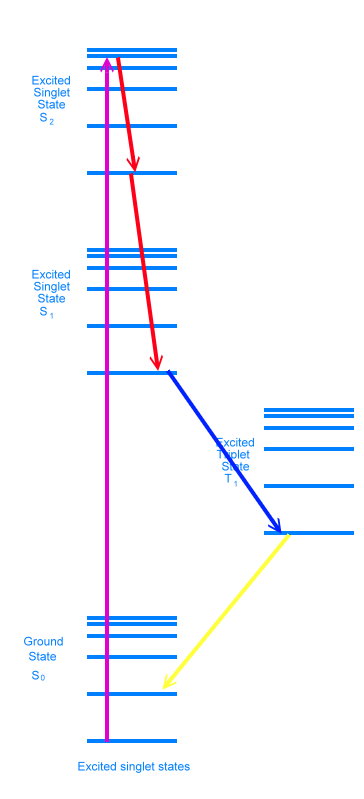
Jablonski diagram for phosphorescence Sometimes the electron will lose energy by flipping its spin, and jump to the excited triplet state. It can then jump back and fluoresce like before, in which case it is called delayed fluorescence. Or it can flip again, and jump to one of the vibrational energy levels just above the singlet ground state. We call this phosphorescence. Flipping electron spins is statistically unfavorable, and this causes a delay in releasing the energy as a photon. Phosphorescent materials glow for a while after the exiting light is removed (glow in the dark paints are phosphorescent). Adding heat energy makes it more likely that electrons will shake loose from their current states and fall down into lower states. Heating a glow in the dark toy will make it appear brighter, but the glow will fade way faster.
Kryptonite
Most glow in the dark products are made from zinc sulfide doped with tiny amounts of copper. This compound is inexpensive, but the glow does not last long. A very long lasting phosphorescent material has recently been discovered. It glows bright green for over 12 hours (all night long) after charging in the afternoon sun for an hour or two. It lasts so long, and glows so brightly, it has been named Kryptonite, after the glowing green mineral in the Superman comics and movies.
Kryptonite in the light You can charge this stuff up by setting it next to a lamp for a while, then turn off the lamp and have a free night-light, or read Superman comics with it under the covers.

Kryptonite in the dark The material is made of strontium magnesium aluminate, doped with the rare earth lanthanides europium and dysprosium. No evil genius should be without a vial of this in his pocket! Imagine sprinkling sand made from Kryptonite onto sidewalks before the concrete hardens. Now you won't need street lights. How many other uses can you think of? Post your ideas on our message board. Make a Kryptonite necklace Next: A high resolution spectrograph Order ultraviolet LEDs and invisible inks, and Kryptonite here. Del.icio.us
- Science Toys
- Magnetism
- Electromagnetism
- Electrochemistry
- Radio
- Thermodynamics
- Aerodynamics
- Light and optics
- Simple laser communicator
- Make your own 3D pictures
- Making permanent rainbows.
- A solar powered marshmallow roaster
- Make a spectroscope from a CD.
- The impossible kaleidoscope
- Make a solar hotdog cooker
- Exploring invisible light
- A high resolution spectrograph.
- Time-lapse photography.
- High speed photography.
- Stacking photos for high depth of field.
- Biology
- Mathematics
- Computers and Electronics
Some of my other web sites:

Send mail to Simon Quellen Field via [email protected]