My friend Theodore Gray introduced me to the hobby of element collecting.
Some people are almost fanatical about it, spending lots of money on many large samples of pure elements from all over the periodic table. But I have found it quite fun to collect samples that are available cheaply from local sources, and it may even be more educational to have samples of the elements in the form that they are most likely to be encountered than in their pure form.
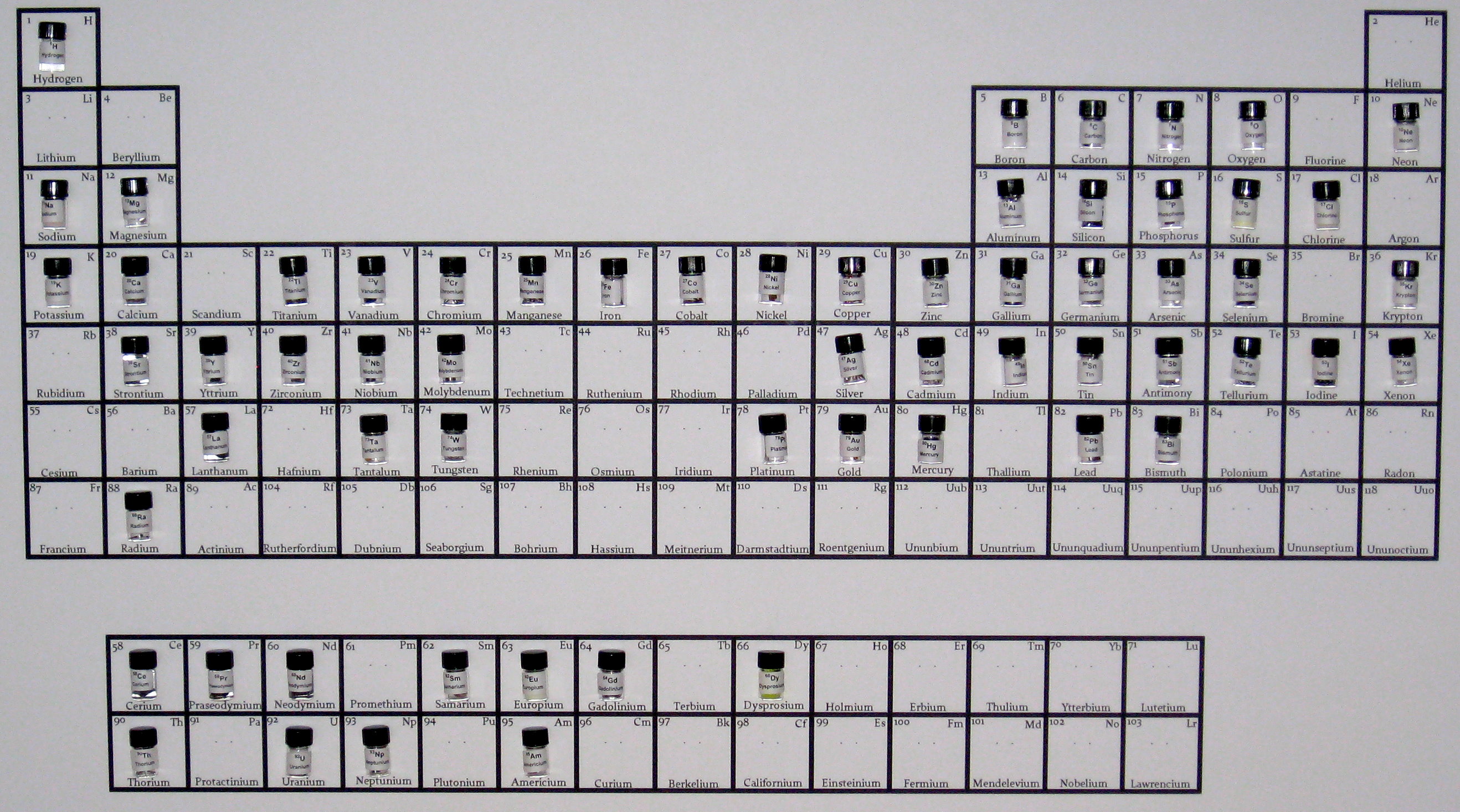
Part of the fun is in filling in the blanks on a fresh empty chart of the periodic table of the elements. Here's how I started out.
First, I made an empty chart with space for little sample bottles.
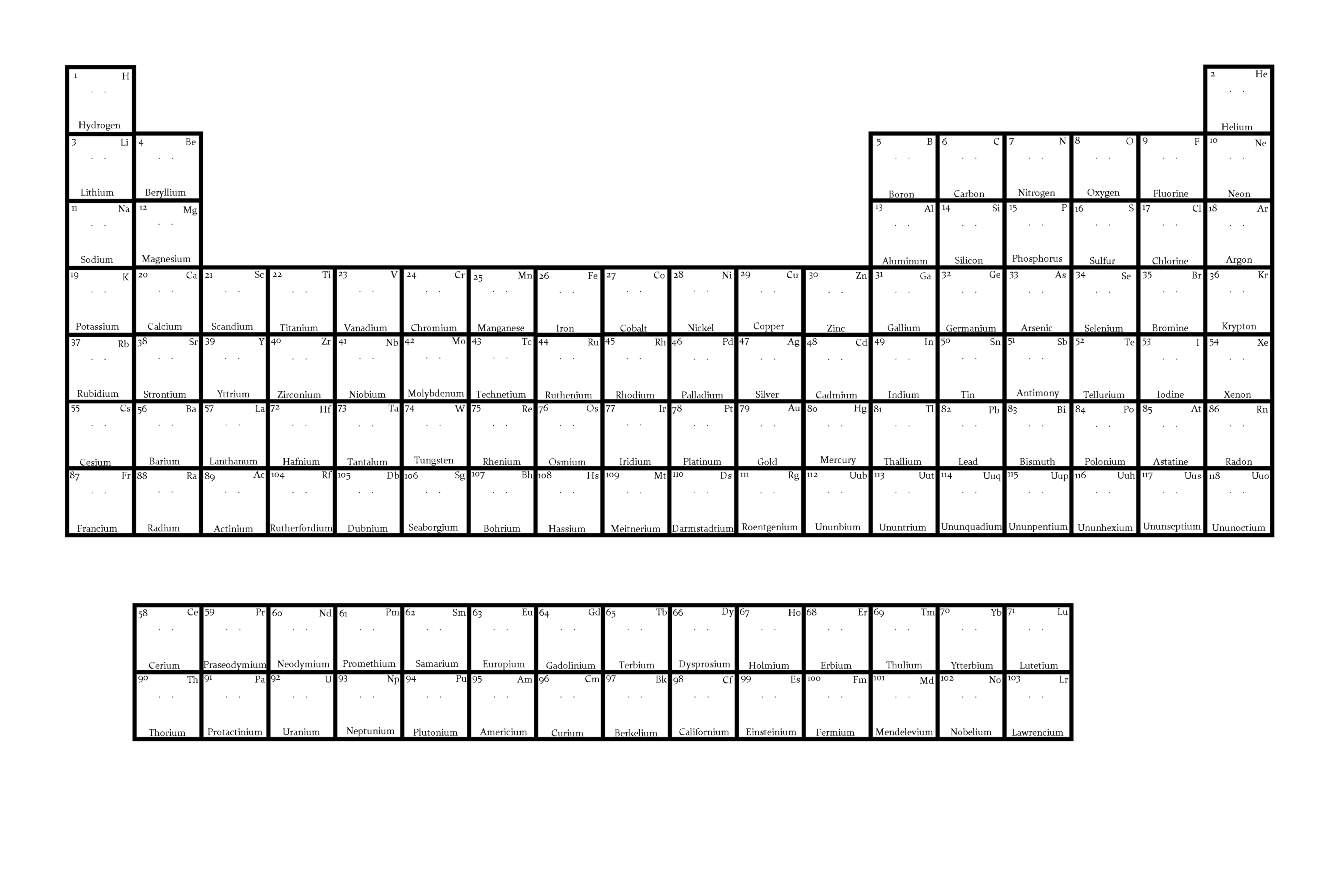
I filled in each square with the atomic number, the symbol, and the name of the element. I also added two small dots in the center of each square. These are there to make it easy to mount the sample bottles. After the chart is printed out on 36 inch by 24 inch paper and mounted on a foam core board, you poke two holes in the board with a push pin, so that you can make a loop of wire to hold the bottle.
If you click on the image above, you will get a huge file that you can save onto a thumb drive and take to your local printer. I took mine to Kinko's, and they printed it out on a huge piece of paper, and mounted that onto the foam core board for me. You can save money by doing that last part yourself.

I hang my sample vials on the board using green enamel coated magnet wire (because I happen to have a lot of that). You can use any convenient wire you have lying around.
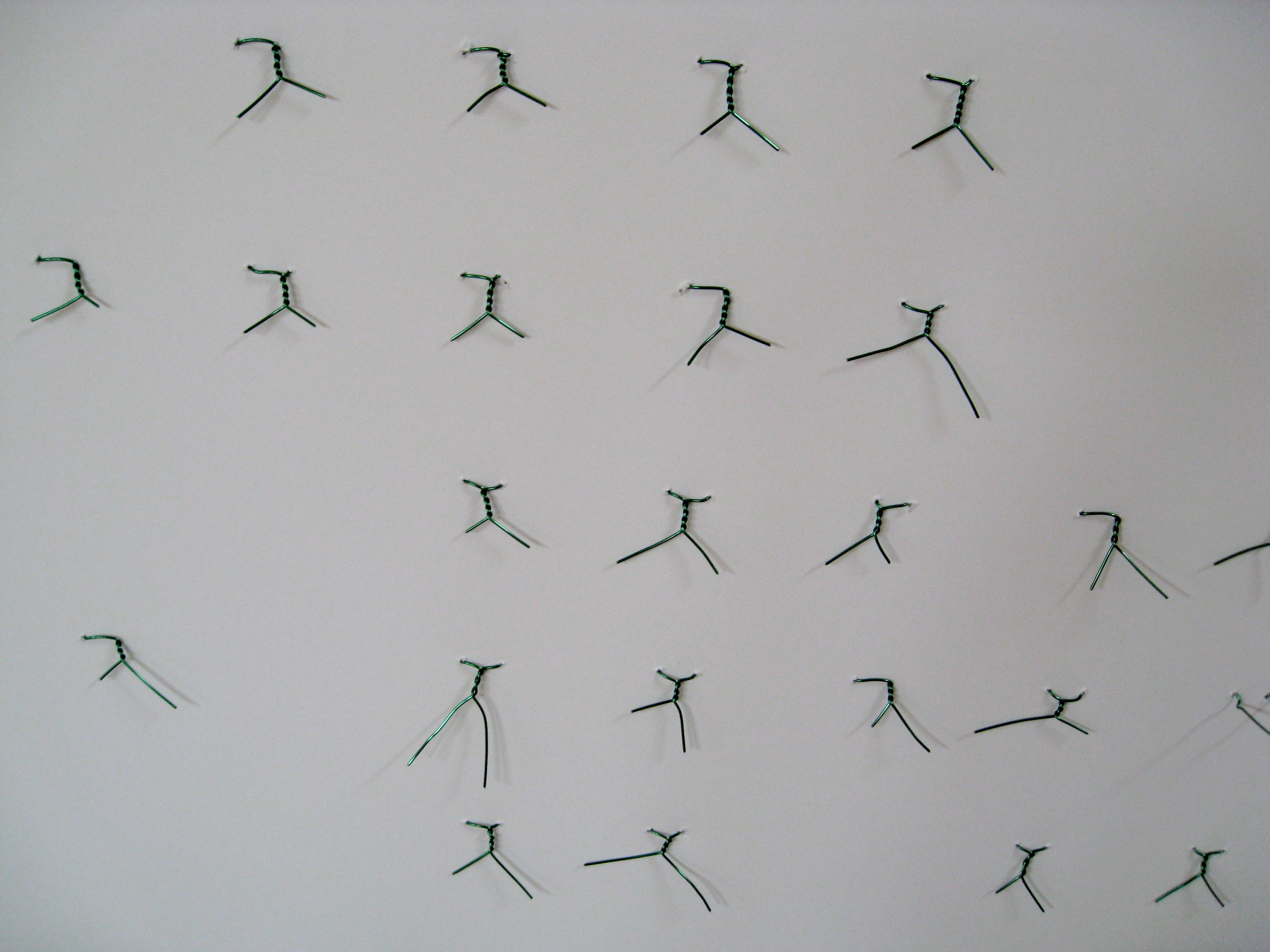
The back of the board shows that the wires are simply twisted to hold the bottles firmly on the board. The wires tighten around the necks of the bottles, and become nearly invisible, while tightly holding onto the bottles so they don't fall off during transport and handling. The wire mounting allows the bottles to be turned, so that either the labels on the bottles face out, or the clear glass faces out, leaving the sample more visible.
I made gummed labels for the bottles, with the atomic number, symbol, and name, so that if the bottles are removed from the board, they can easily be put back in the right spot. To make it easier for our readers, I have added both the sample bottles and the lables to our catalog. If you need the green enamel coated copper wire, you can get it there as well.
Obtaining Americium and Neptunium

Inside inexpensive smoke alarms is a tiny amount of the radioactive element Americium. The isotope used, americium 241, has a half life of 452 years. Since americium 241 decays into the much more stable isotope neptunium 237 (half-life 2.1 million years), the sample in the smoke detector will have a few trillion new neptunium atoms in it every year.
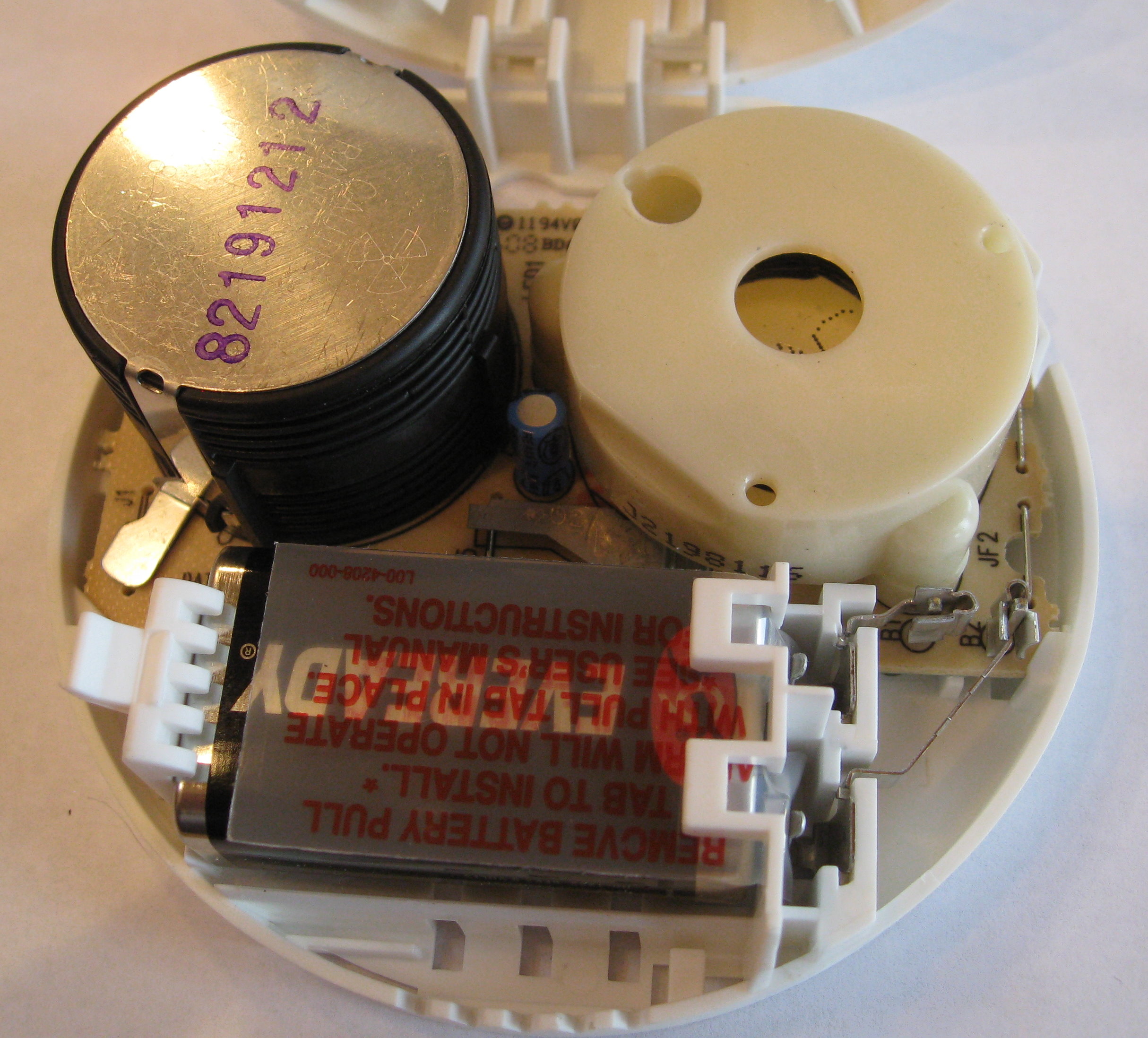
To get to the sample, we disassemble the smoke detector.
The chamber that contains the americium sample is usually easy to find and open.
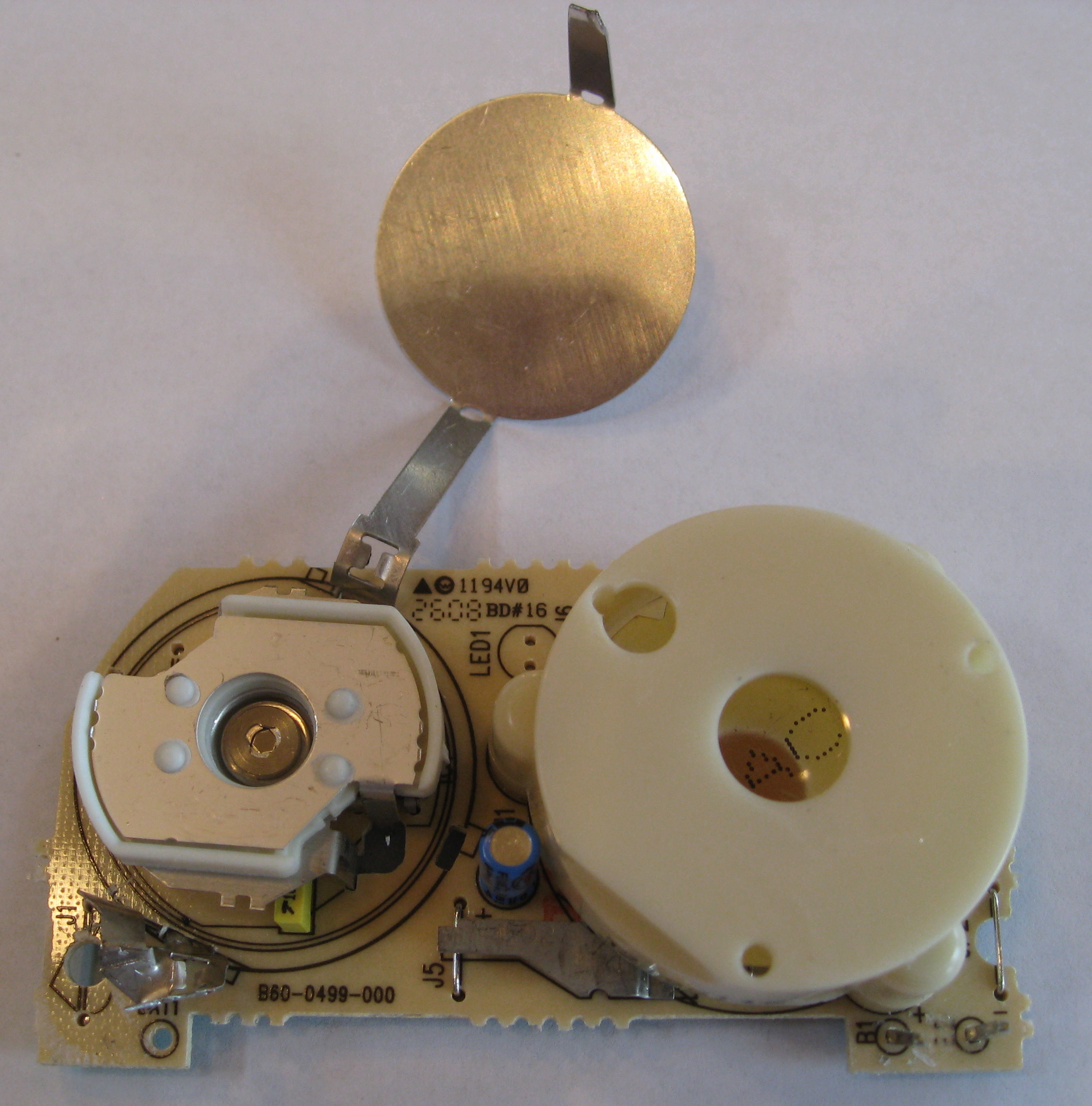
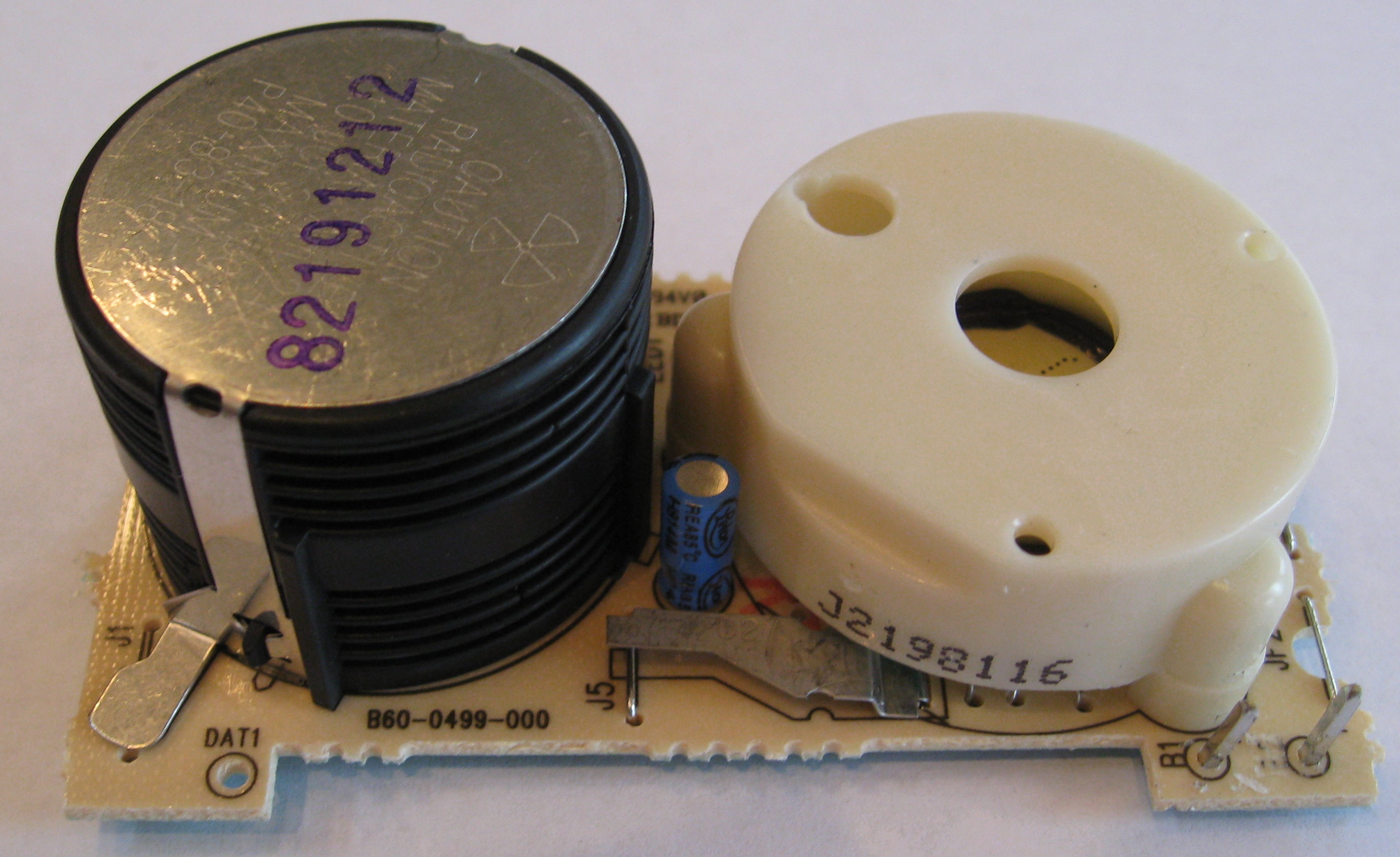
Removing the plastic parts gets us closer to the sample.
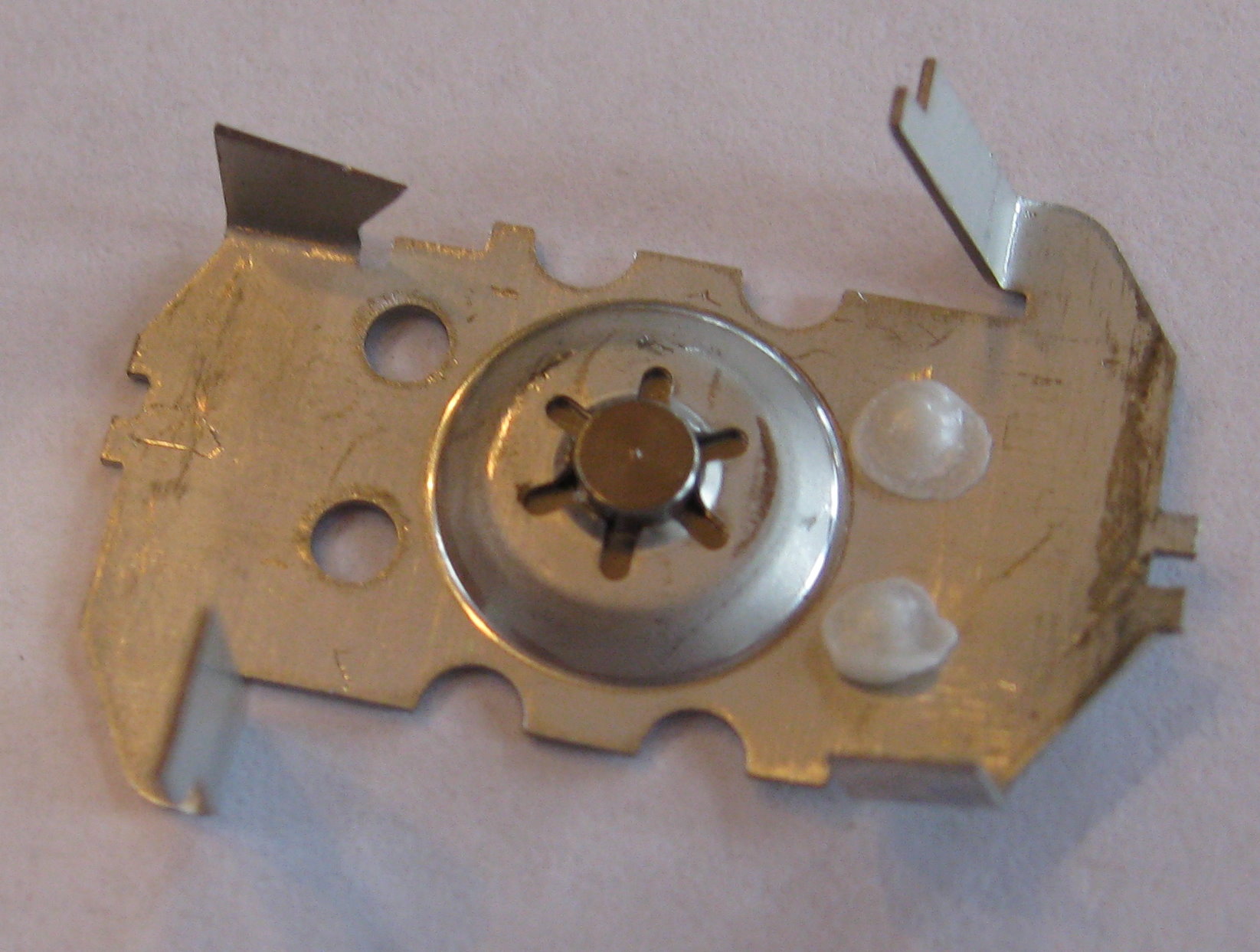
The sample itself, in this smoke detector, is plated onto a small button of metal. Other detectors I have disassembled have the americium plated onto a small disc.

If you have a geiger counter or a scintillation detector, you can use it to confirm that the sample is radioactive. Since americium 241 emits only alpha particles (and a very small amount of low energy gamma), it is safe if kept in the glass bottle, since alpha particles don't penetrate glass.
Elements from a hardware store
Hardware stores carry many items that have either pure elements, or compounds of otherwise hard to find elements. Lighter flints contain cerium oxide, battery charging posts contain lead, and halogen bulbs contain nice thick tungsten filaments (as well as bromine or iodine, although they are seldom labelled in such a way that you can tell which is used).













Making a game out of it
If you're at all like me, your collection will first be about filling in the blanks any way you can. The vial containing the sample for Sodium in my collection has salt in it. Salt is sodium chloride, and that is one of the main forms people find sodium in as they go about their daily lives. Likewise, the vials for Nitrogen and Oxygen are just full of air.
Over time, you may come across samples of the elements in their pure forms. My sample of Silver is just some pieces of lead-free solder from a hardware store, which contains 3% silver. It will not be difficult to replace that with pure silver wire from a jewelry supply store.
Since some forms of the elements are easier to find than others, my friends and I have found it useful to make a list of the elements with points assigned to them based on the degree of difficulty in obtaining the sample. Salt, and Air, and Aluminum foil are arbitrarily assigned one point. Silver bearing solder is worth five points, while pure silver is worth twenty five points. The whole list of where to find elements and how many points each sample is worth is shown at the end of this page.
Scoring points
In the table below, mark which samples you have collected by clicking in the check box beside the description. The total number of points will be calculated for you.
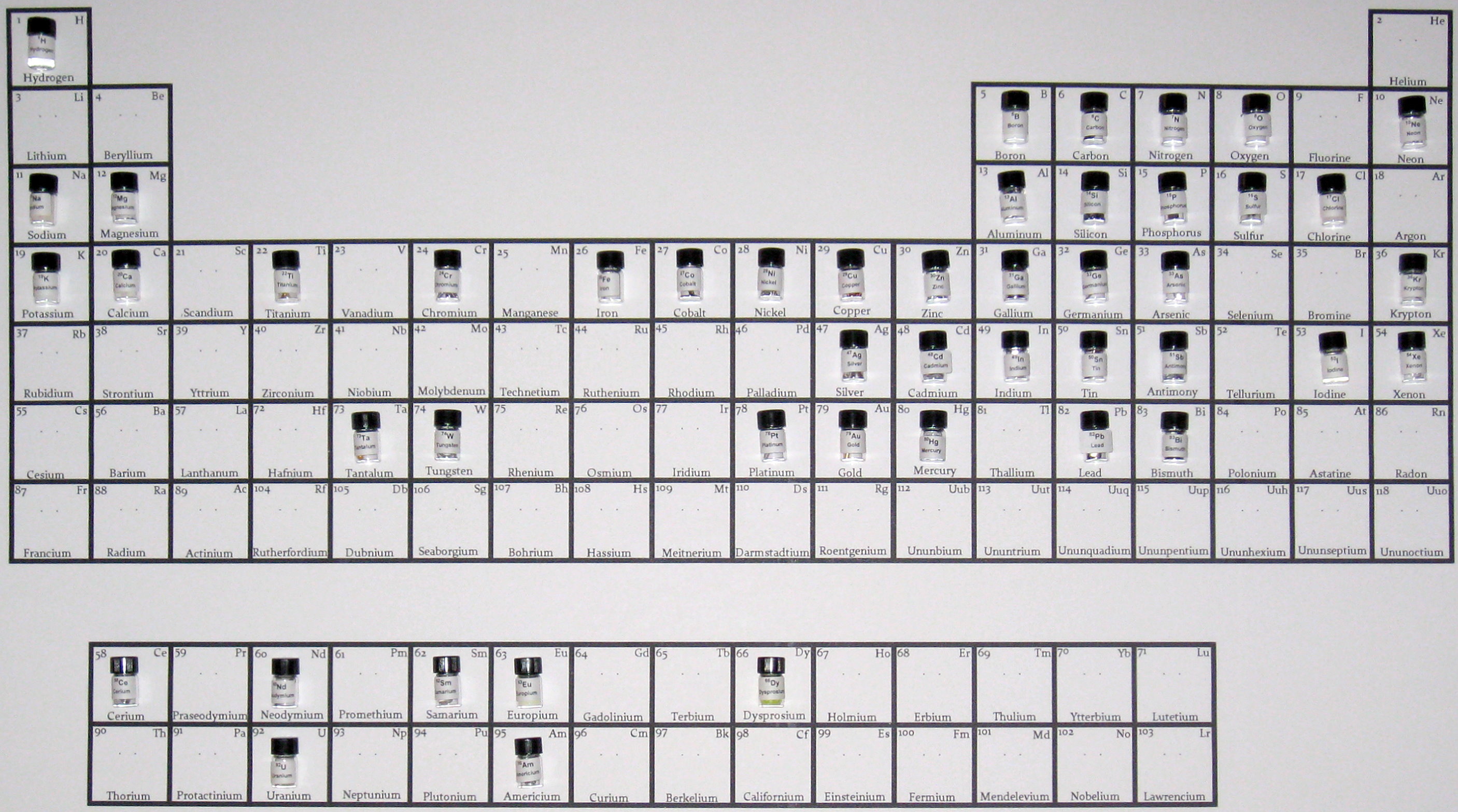
After a few weeks, my collection looked a little more filled-in: