Pierre-Joseph Pelletier was a chemist and director of the School of Pharmacy in Paris in 1837. Famous for earlier work, where he was the first to isolate chlorophyll, quinine, and strychnine, along with several other plant alkaloids, he was a frequent collaborator with the Polish chemist Filip Walter.
In that year (1837) the two were distilling pine oil, and isolating it constituents. One of those was a substance new to science, the molecule toluene.
Toluene is a good starting point for more complex molecules. It's like a benzene ring with a handle, and the handle helps when adding toluene to other molecules to make whatever the organic chemist is trying to make.
In 1863, the German chemist Julius Wilbrand was trying to make dyes. An important class of dyes at the time was the aniline dyes, made from the molecule aniline. The Russian chemist Nikolai Zinin (Alfred Nobel's childhood tutor) had made aniline from nitrobenzene by adding hydrogen in 1842. So, it was natural to suspect that nitrating toluene might make a good start at creating a new type of dye. After all, picric acid was a yellow dye, and using toluene instead of phenol seemed a reasonable substitution.
Adding an NO2 group to toluene is fairly simple. Adding another NO2 group is a little bit harder. Adding a third NO2 group is yet another step. In the second and third steps, the groups can end up on adjacent carbons, or they can be arranged symmetrically to form the molecule 2,4,6-trinitrotoluene, which is the molecule Wilbrand came up with. It makes a nice pale yellow dye. Similar molecules are used today in hair dye products.
It would be natural to investigate the uses of the new molecule as an explosive. A very similar molecule, 2,4,6-trinitrophenol (known as picric acid) was already in use as an explosive, as was trinitroglycerol (another name for nitroglycerin). But trinitrotoluene has a high activation energy, making it difficult to detonate, and it is less powerful than picric acid or nitroglycerin. Moreover, it is toxic.
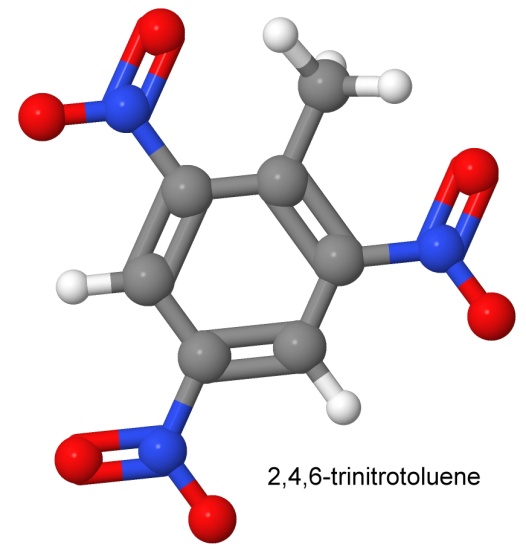
The difficulty in detonating TNT became an advantage when German munitions manufacturers wanted to fill shells with an explosive that would not detonate until after the shell had pierced the armor of enemy defenses. Add to this its insolubility in water, and the ease of melting it (it melts at 80° Celsius without decomposing) to pour into shells and bombs, and TNT's drawbacks start to look like less of a problem.
Still, it was 1902 before German artillery shells were using TNT, and 1907 before the British started using them in shells. To make TNT less expensive, and to stretch supplies during wartime, it was mixed with ammonium nitrate to make the explosive amatol.
TNT makes large amounts of black smoke when it detonates, because there is not enough oxygen in the molecule to completely oxidize all of the carbon atoms. The result is sooty smoke. Adding ammonium nitrate, and explosive in its own right, corrected the oxygen balance, allowing those carbon atoms to react and add to the explosive force. However, the major benefit of ammonium nitrate was that it was cheap and plentiful. If the amount of TNT in the amatol stayed above 60%, there was little reduction in the power of the explosive, despite the fact that ammonium nitrate is less powerful.
Oxygen balance is the term for indicating how well matched the fuel and oxidizers are in an explosive. If all of the carbon, hydrogen, and metals in a molecule are completely oxidized, then the oxygen balance is zero. TNT has an oxygen balance of -74%, showing that is has insufficient oxygen. Since the remainder of the molecule is mostly carbon, the thick black sooty smoke of a TNT explosion is the result.
When an explosive detonates, we can generalize the reaction products based on what happens to the oxygen. Whatever the actual chain of reactions might be, a rule of thumb is to take the reactants one-by-one with the following rules:
- Combine all the nitrogen atoms to form N2.
- Then burn all the hydrogen with any oxygen to form H2O.
- Next, use whatever oxygen remains to burn carbon to CO (carbon monoxide).
- If oxygen remains, burn CO to CO2 (carbon dioxide).
- Lastly, any remaining oxygen produces O2 (molecular oxygen gas).
Ammonium nitrate has an oxygen balance of +20%, so it releases extra oxygen when it explodes. But the velocity of detonation for ammonium nitrate is low, and a mixture of 80% ammonium nitrate with 20% TNT has a detonation velocity of 5,130 meters per second, compared to 6,924 for pure TNT. The actual detonation velocity of an explosive varies with the size of the particles, whether any solvents remain in the mix, whether the product is cast or cold pressed, and the size of the charge, among other considerations. The velocities given by one test will differ by as much as 300 meters per second from another, under different circumstances.
The 80/20 mix of amatol has an oxygen balance of +1. An "ideal" oxygen balance would be zero. When comparing explosive power to pure TNT, however, the 80/20 mix is 30% more powerful. Thus, we can see that simple oxygen balance is not all there is to know about an explosive.
What balancing the oxygen does for amatol is increase the amount of gas that was produced. So while the brisance (the ability to shatter) is reduced, due to the lower velocity, the ability to move material (the heave) is increased.
In mining, sometimes the rock is hard, requiring a brisant explosive, and sometimes it is softer or porous, in which case, an explosive with less shattering ability and more ability to heave the rock out of the way is desired. We can think of brisance and heave in terms of a lever — how hard you have to push can be traded for how far you have to push.
Adding tiny flakes of aluminum to amatol give the explosive ammonal. The aluminum helps to balance the oxygen, using up the extra oxygen the ammonium nitrate provided to amatol. It also burns exceedingly hot, and produces a bright flash at night, helping the artillerymen to adjust their aim. During the day, ammonal produces white smoke, which can also be seen from a distance and used for aiming adjustments.
Ammonal was used effectively in World War I. In the Battle or Messines, for example, British engineers tunneled under German front lines and planted over 13 tons of ammonal explosive, which created 19 large craters and killed an estimated 10,000 German troops.
The relative explosive power is a function of the energy released in the explosion, multiplied by the amount of gas produced, divided by the square of the weight of the explosive. To make comparisons easier, it is now common to compare explosives to TNT. Since the comparison is done by weight, the density of the explosive becomes significant. To equal the force of one kilogram of TNT, for example, you would need about 2.4 kilograms of ammonium nitrate.
Some relative effectiveness values for a few explosives:
|
Ammonium nitrate |
0.42 |
|
Black powder |
0.55 |
|
ANFO |
0.74 |
|
TATP |
0.80 |
|
50/50 Amatol |
0.91 |
|
TNT |
1.00 |
|
80/20 Amatol |
1.10 |
|
Nitrocellulose |
1.10 |
|
Picric Acid |
1.20 |
|
C4 |
1.34 |
|
Nitroglycerin |
1.54 |
|
RDX |
1.60 |
|
PETN |
1.66 |
|
HMX |
1.70 |
|
Octanitrocubane |
2.38 |