In 1877, German chemists Wilhelm Michler and Carl Meyer invented the compound tetryl, or 2,4,6-trinitrophenylmethylnitramine. It took until 1886 before its structure was established by Dutch chemist Karel Hendrik Mertens. Another Dutch chemist, Pieter van Romburgh later (in 1889) proved the structure by synthesizing it from picryl chloride and potassium methylnitramine.
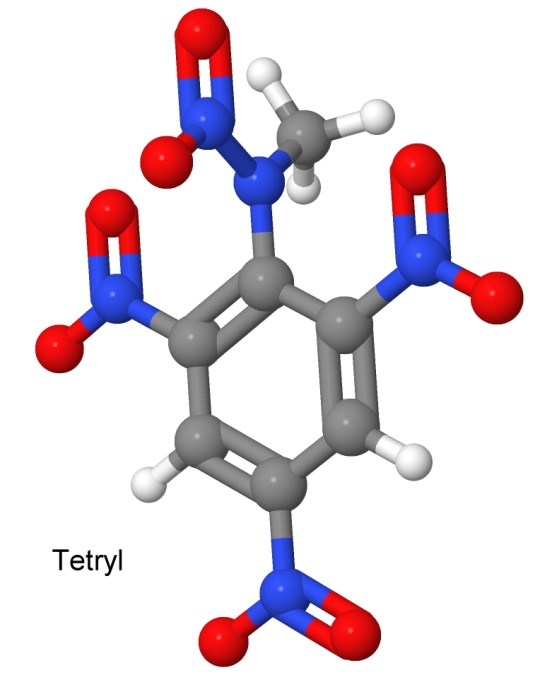
Tetryl was the first of the nitramine class of high explosives, which includes many of the most explosive compounds currently in use. Others in this class include RDX and HMX, developed much later. Nitramines are characterized by the two nitrogen atoms connected together, with one of them then connected to two oxygen atoms. Tetryl has one nitramine group, shown at the top of the drawing.
Tetryl is about as sensitive to shock and heat as picric acid, making it a good booster, but generally not as safe as TNT or ammonium picrate. As a booster, it is used in blasting caps, with primary explosives such as mercury fulminate and potassium chlorate to set it off. The tetryl then quite reliably sets off the main charge of TNT or other less sensitive explosive, due to the very high detonation velocity and brisance of tetryl.
Tetryl can be detonated by a spark or by friction, but is almost always detonated by a primary explosive in actual use.
Tetryl was used in both World Wars as a booster, and by itself in some of the smaller caliber shells. It was more expensive than TNT and Amatol, but only small quantities were needed, since it was not often used as the main charge. Small quantities is a relative term — in World War I the U.S. alone used a million and a half pounds up to September 1st of 1918, and contracted for 1,432,000 pounds to be delivered by December 31st of that year. Prior to the war, the U.S. production of tetryl was negligible.
A mixture of 70% tetryl and 30% TNT is called tetrytol. As a booster, tetrytol is safer to use than tetryl, being less sensitive to shock, heat, and friction, and it has the ability to be cast into shaped charges. It was used in burster tubes for nerve gas weapons. It was also less expensive than pure tetryl.
Tetryl was more difficult (and hence more expensive) to produce than other common explosives. Many steps were involved in the production, and many by-products are produced during its manufacture that were difficult to remove. The history of tetryl is thus one of continual search for better and cheaper ways to manufacture it, all the way up to its replacement by RDX and HMX. (Tetryl is no longer manufactured in the U.S.)
The long chain of reactions that results in tetryl starts with methyl alcohol. At the time of the First World War, methyl alcohol (called wood alcohol) was exclusively made from the distillation of wood. Wartime production of methyl alcohol required more wood than was available, so the supply of methyl alcohol was limited.
The vapor of methyl alcohol was passed over a hot copper mesh, which acts as a catalyst for the production of formaldehyde from the vapor. The reaction is hot enough that the copper mesh stays red hot, and only has to be heated at the start.
The formaldehyde is then used to make methylamine (which is also a starting material for RDX and HMX). Methylamine is converted to dimethylaniline, by a multi-step process, and then that can be nitrated to form tetryl. The problem with this synthesis (besides the need to start with methyl alcohol) is that many by-products of the reactions accumulate, and require difficult and wasteful purification.
A better method, where methyl alcohol is reacted with ammonia by applying heat with a thorium oxide catalyst to form methylamine, which then reacts with dinitrochlorobenzene to form 2,4-dinitromonomethylaniline, was developed. The final product is easily nitrated to tetryl without byproducts.
Despite the new method, methyl alcohol was still the starting ingredient, and was in short supply during World War I.