Homemade batteries are a popular subject with my readers. Making electricity from things you find around the house is a fun project.
There are lots of easy ways to make homemade batteries. Basically, any two different kinds of metal can be placed in a conducting solution and you get a battery. Familiar homemade batteries include sticking copper and zinc strips into a lemon or a potato to make a battery.
One quick battery is made from a soda can, the soda from the can, and some copper.

The photo above shows a battery made by placing a strip of copper and a strip of aluminum into a glass of Coca-Cola (I used the sugar-free cherry flavored variety because that's what I found in the refrigerator).
You can make the aluminum strip by cutting open the can. You will need some sandpaper to sand off the paint and plastic coating from the aluminum before using it. Or you can get strips of aluminum already free of coatings from a hardware store, or from our catalog.
You can get copper flashing from a hardware store and cut out a strip of it, or you can use a bunch of copper wire (the more surface area exposed to the liquid, the more electrical current is produced). Or, as before, you can get pre-cut strips from our catalog.
The aluminum-copper-coke battery will produce about three quarters of a volt.

Using a zinc strip instead of the aluminum produces a little over a volt in the copper-zinc-coke battery. That zinc should work better than aluminum in a battery is a little surprising, since aluminum is normally more reactive than zinc, but in this cell I suspect the aluminum has an oxide coating that is interfering with the reaction. I have not seen a thorough scientific study of a Coca-Cola copper aluminum battery, and it may be a while before I do.
You can get zinc strips from our catalog.

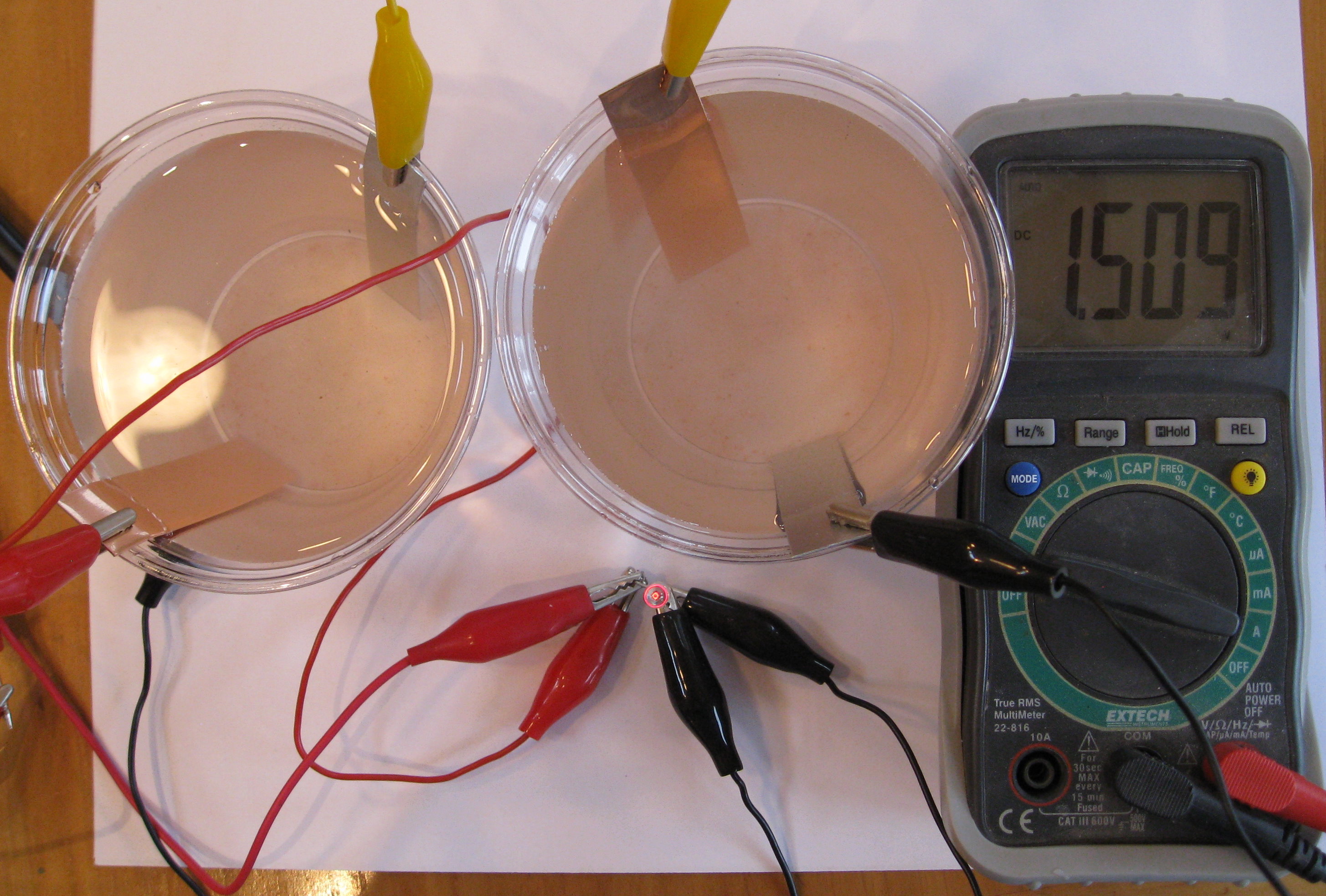
Another easy battery to make at home is the zinc-air battery. In this battery, the strip of zinc is oxidized by dissolved oxygen in salt water. We used a level tablespoon of salt in a cup of water.
The "batteries" we have shown here are more correctly called "cells". An actual battery is made up of two or more cells.
The zinc-air cell produces about three quarters of a volt. To get higher voltages, which are needed to run things like light emitting diodes (LEDs) or calculators or watches, we connect two or more cells in series to make a battery.
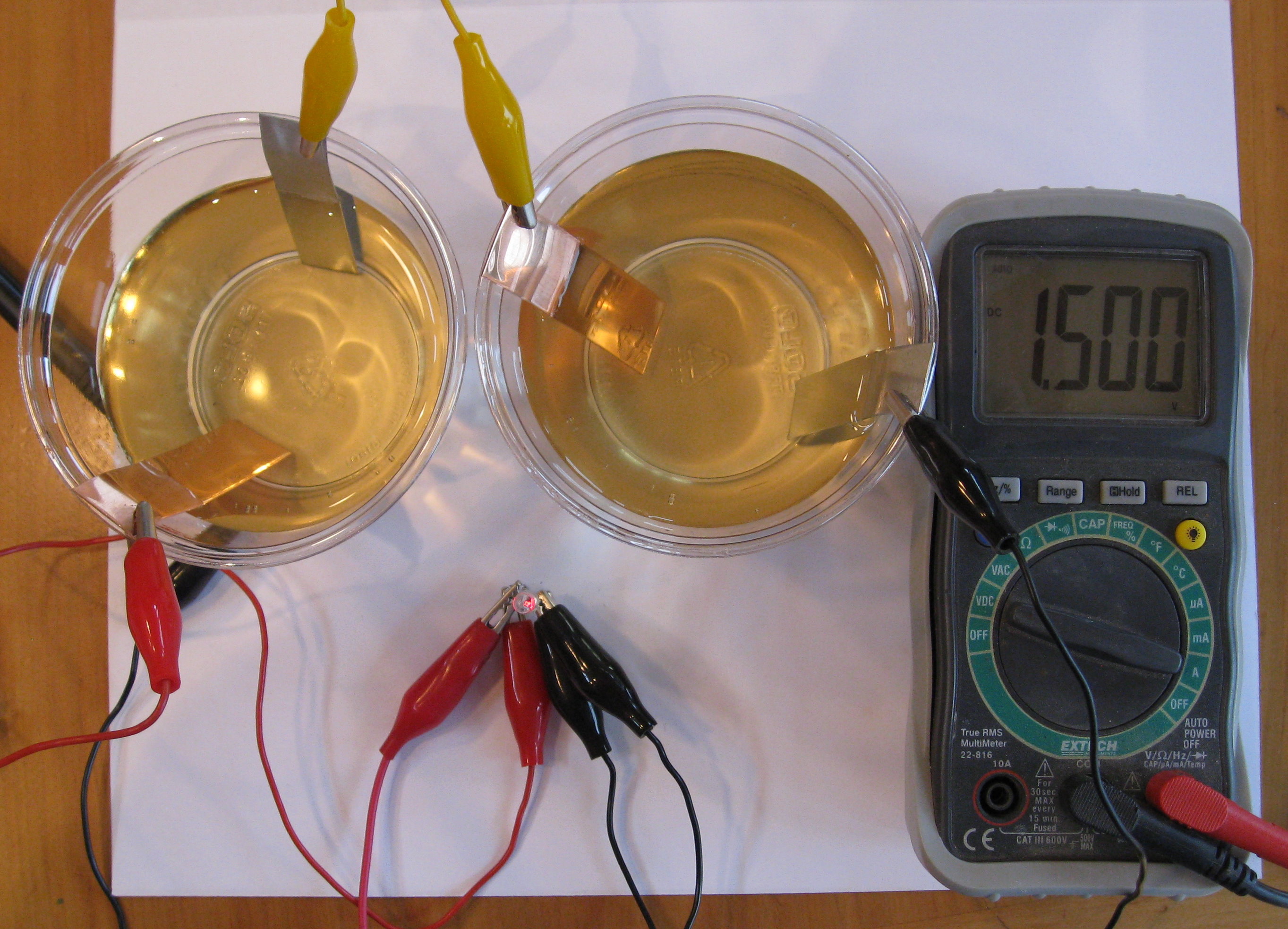
Two zinc-air cells in series produce about a volt and a half. In the photo above, you can see that this is enough to make a red LED light up. To make it brighter, you can add more cells.
If the LED in your project doesn't light up, try reversing the leads. The diode is a device that only works in one direction. The leads on the diode usually have one lead longer than the other, to make it easy to see which way it is connected. With my diode, the long lead was the one to connect to the copper electrode.

Another way to make the cell produce a higher voltage is to change the chemistry. The zinc-air cell produces more voltage in the photo above because we added some hydrogen peroxide to the salt water. You can also add chlorine bleach, but hydrogen peroxide is safer and has no odor.

If you don't have a strip of zinc, you can make an aluminum-air battery, as shown in the photo above. It produces a little more than half a volt, so you will need three cells in series to light the LED.
A different chemistry is involved in the copper-zinc-vinegar battery, shown in the photo above. In this battery, the zinc is oxidized by copper ions from the copper strip. In this battery, the copper gradually migrates into the vinegar, and then replaces the zinc at the zinc electrode.

After lighting the LED all night long, you can see a black coating of copper and copper oxide sludge has formed on the zinc.
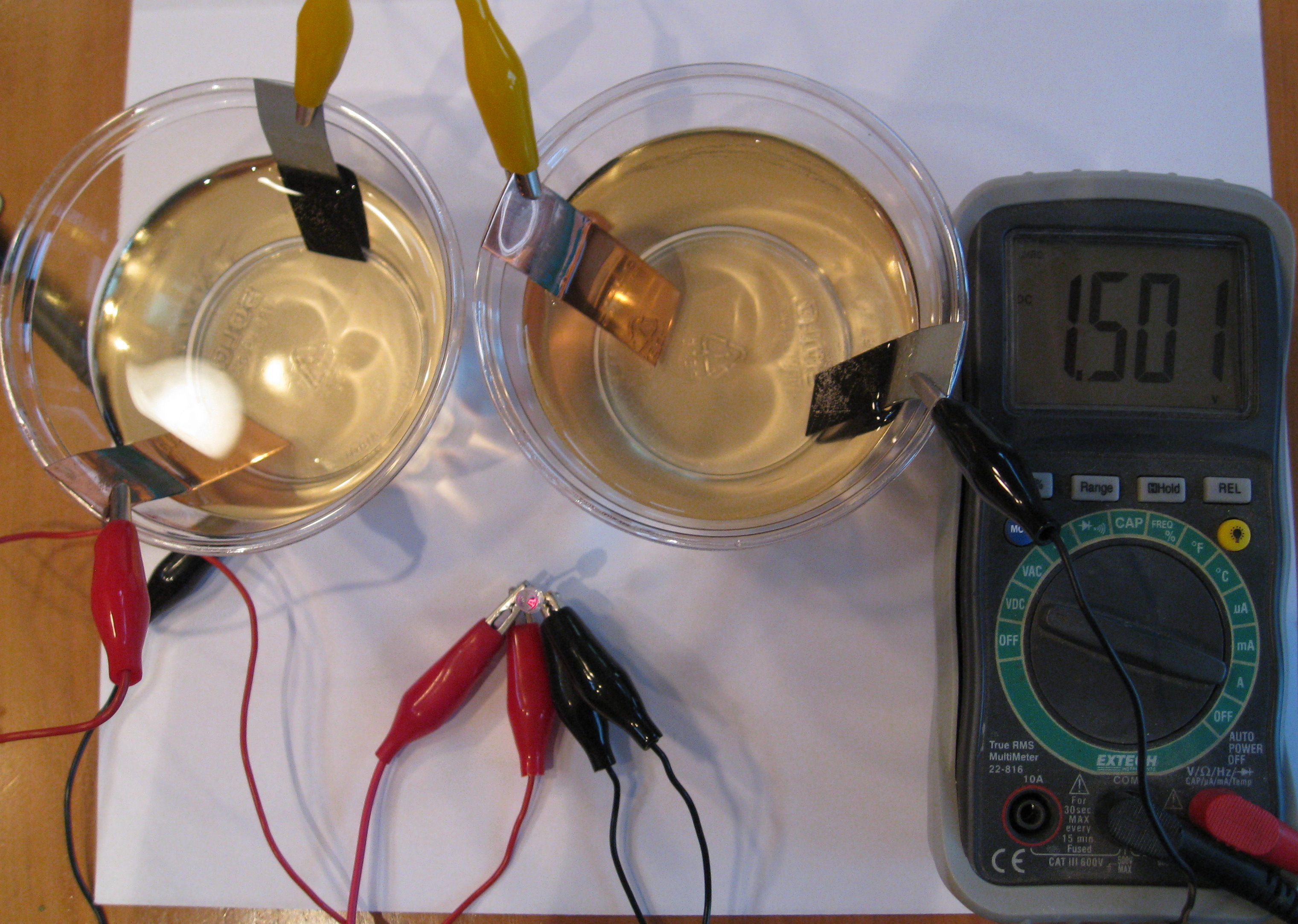
Even though the battery has been lighting the LED all night, it still has a lot of zinc left to keep it lit for several days.
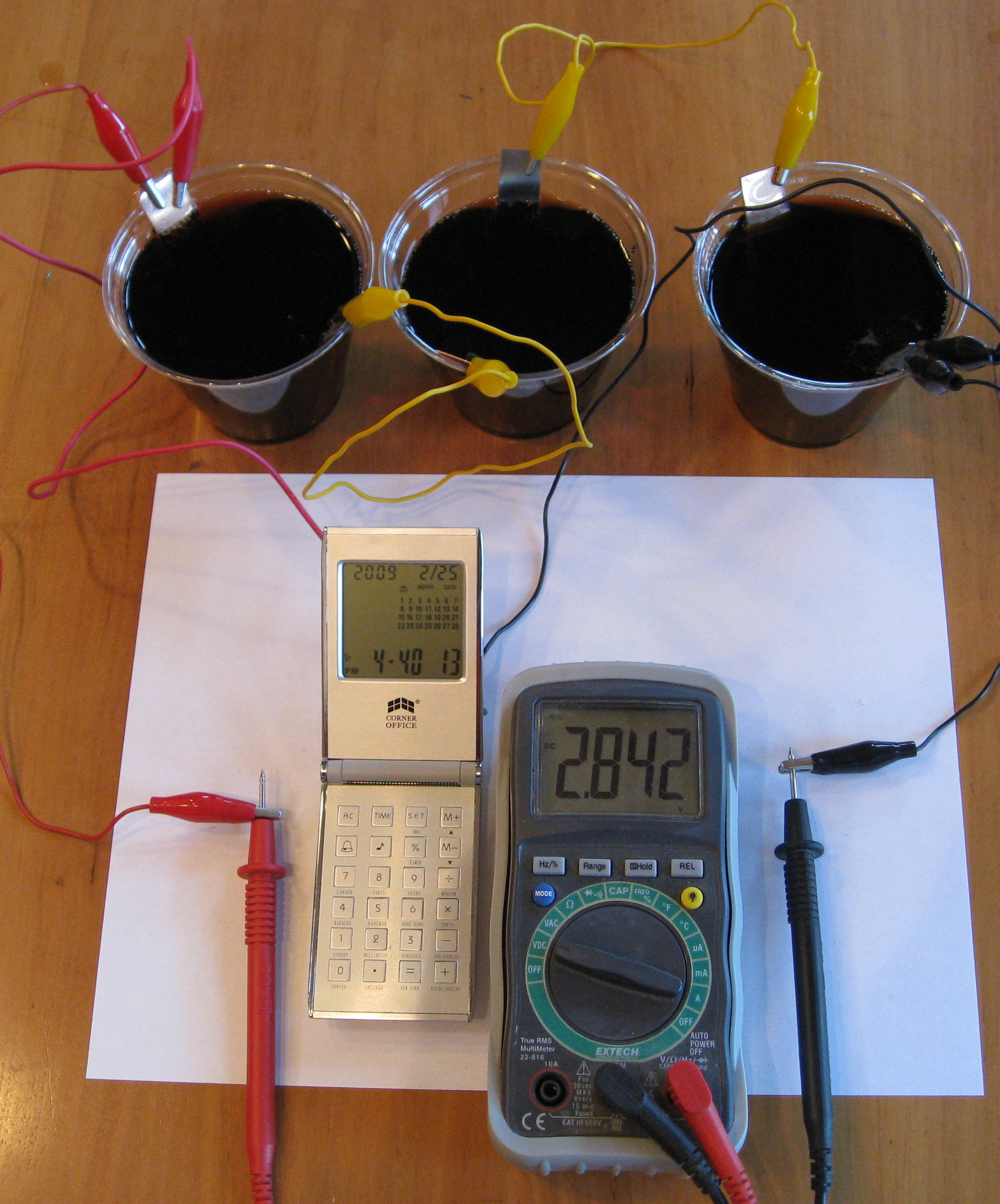
Three of the copper-zinc-coke batteries produce about 3 volts, and can replace the 3 volt lithium battery in this small clock/calendar/calculator. It has been running for days now, and can probably last months. The calculator works, and the alarm chimes play just fine.
How does it do that?
Metal atoms are held together by electrical attractions between the nuclei and the electrons around the atoms.
When you place a strip of metal in a glass of water, the water molecules interact with the metal atoms on the surface of the strip. Water molecules are polar, meaning the one side is slightly positive, and the other side is slightly negative. This is because the two hydrogen atoms are not on opposite sides of the oxygen atom, but are instead about 105° apart. The hydrogen side is positive, and the oxygen side is negative.
At the interface between the water and the metal, some of the metal nuclei are attracted to the negative side of the water molecules. This attraction makes it easier for a metal nucleus to leave one or more of its electrons behind in the metal strip, and migrate away from the strip into the water.
The strip is left with a very small negative electric charge, because it now has one less positive nucleus in it. This tiny charge does not pull very much on the metal ion that has left the strip. In fact, that ion (a metal atom with one or more electrons missing) is quickly surrounded by water molecules, whose negative side is attracted to the positive metal ion. This blanket of water molecules spreads out the positive charge over a larger area, making it even less attracted to the metal strip.
This is a very temporary effect, and the metal ion usually gets attracted back to the strip very quickly. But since there are enormous numbers of atoms at the surface of the metal strip, and an enormous number of metal ions are in the water at any given time, the metal strip ends up with quite a few more electrons than metal nuclei. This gives the strip a slight negative charge.
Some metals hold on to their atoms more tightly than others. This is why different metals have different boiling points (and to a lesser extent, it explains why they have different melting points, although melting is more complicated, and other effects come into play).
This means that some metal strips will become more negative when placed in water than others do.
If one metal strip has more extra electrons than another one does, those electrons will flow from the first strip to the second, until they both have the same charge. But to flow, the electrons need a conductive path. We give them that path when we connect two strips of different metals with a wire. The electrons then flow through that wire, creating an electric current.
Copper, zinc, and acid
In the case of the copper and zinc strips, the copper holds onto its atoms more strongly than the zinc does. The zinc strip is therefore more negative than the copper strip, and the electrons flow from the zinc to the copper.
When the forces are eventually balanced, the copper strip ends up with more electrons than the zinc strip. The zinc strip now has fewer electrons, and it cannot attract the zinc ions back to the strip.
If our battery just had water in it, not much more would happen. But our Coca-Cola battery has water plus phosphoric acid. Our vinegar battery has water plus acetic acid. An acid is something that has an easily detached hydrogen ion. Hydrogen ions are positive, and the remaining part of the acid becomes negative when it loses the hydrogen ion. In our two batteries, the remaining parts are the phosphate ion and the acetate ion, respectively.
So what happens when all of those positively charged zinc ions bump into those negatively charged phosphate ions? They phosphate ion is more strongly attracted to the zinc ion than to the hydrogen ion. The positively charged hydrogen ion is attracted to the copper strip, because the copper strip has the extra electrons, and is thus negative (opposite charges attract).
The hydrogen ions attract the electrons from the copper, and become neutral hydrogen atoms. These join up in pairs to become hydrogen molecules, and form bubbles on the copper strip. Eventually the bubbles become big enough to float up to the surface and leave the system entirely.
Now the copper strip no longer has the extra electrons. It attracts more from the zinc strip through the connecting wire, as it did when we first connected the wire.
The copper ions next to the copper strip are not as attracted to the strip as they were before. The hydrogen ions keep taking the electrons that attracted the copper ions. So those ions are free to move through the liquid.
At the zinc strip, zinc ions are being removed, leaving extra electrons. Some of those electrons travel through the wire to the copper strip. But some of them encounter the copper ions that happen to bump into the zinc strip. Those ions grab the electrons, and become copper atoms. We can see those atoms build up on the zinc strip. They look like a black film, because the oxygen in the water combines with the copper to form black copper oxide.
Eventually, all of the zinc is eaten up, and the copper and copper oxide falls into a pile beneath where the zinc strip used to be. The battery is now dead, and no more electrons flow through the wire. If there was not a lot of acid in the water, it may be the first thing to be used up, and the battery may die while there is still some zinc left on the zinc strip.
Copper, zinc, and salt
A different chemistry happens when we have salt instead of acid in the water.
Salt breaks up in water to make positive sodium ions and negative chloride ions. These ions reduce the energy needed for water to split into hydroxide ions (OH-) and hydrogen ions H+ (the hydrogen ions quickly find another water molecule and create hydronium ions, H3O+).
At the zinc strip, the zinc ion combines with four hydroxide ions to form one ion of zincate (Zn(OH)42-), leaving two electrons behind on the zinc strip. The chlorine ions from the salt then combine with the hydronium ions left over when the hydroxide ions were taken away by the zinc, and form hydrochloric acid.
Over on the copper strip, four electrons combine with oxygen dissolved in the water and two molecules of water to form four hydroxide ions. The sodium ions from the salt combine with these hydroxide ions to make sodium hydroxide.
The hydrochloric acid and the sodium hydroxide combine back into salt. So the salt is merely in the picture as a way to move charges through the water. It is not used up.
We can summarize what happens at the zinc strip (called the anode this way:
| Zn + 4OH- | ⇒ | Zn(OH)42- + 2e- |
| 4Cl- + 4H2O | ⇒ | 4HCl + 4OH- |
| Zn(OH)42- | ⇒ | ZnO + H2O + 2OH- |
At the copper strip (called the cathode) we have:
| O2 + 2H2O + 4e- | ⇒ | 4OH-. |
| 4Na+ + 4OH- | ⇒ | 4NaOH |
Now we can see why it is called a zinc-air battery. The oxygen from the air is combining with the zinc. The copper electrode is just there to conduct the electrons, and does not participate in the chemistry. It can be replaced with a carbon rod.
You may notice that after a short while, the oxygen in the battery is used up, and the current (and thus the brightness of the LED) begins to drop. Stirring the salt water helps to put more oxygen in the water, and the LED gets bright again.
Copper, aluminum, and salt
The aluminum air battery is very similar to the zinc air battery.
At the zinc anode:
| Al + 3OH- | ⇒ | Al(OH)3 + 3e- |
At the copper cathode:
| O2 + 2H2O + 4e- | ⇒ | 4OH-. |
As before, the sodium and chloride ions help to move the charges.
In the aluminum air battery with salt water, the voltage is not as high as it could be. Using potassium hydroxide instead of salt water brings the voltage up to 1.2 volts per cell by reducing the effects of the oxide layer.