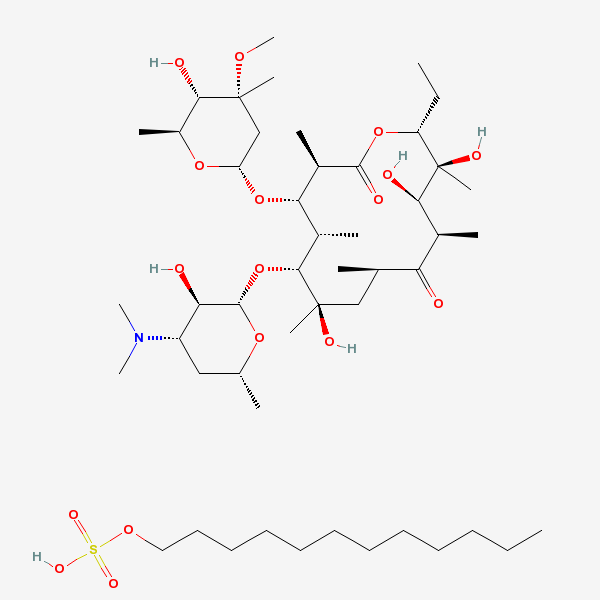
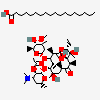
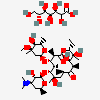
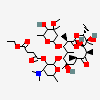
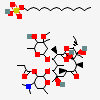
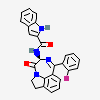
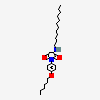
Lauryl sulfate propionyl erythromycin ester
 PubChem Notes:
PubChem Notes:
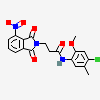
Erythromycin Estolate A macrolide antibiotic, produced by Streptomyces erythreus. It is the lauryl sulfate salt of the propionic ester of erythromycin. This erythromycin salt acts primarily as a bacteriostatic agent. In sensitive organisms, it inhibits protein synthesis by binding to 50S ribosomal subunits. This binding process inhibits peptidyl transferase activity and interferes with translocation of amino acids during translation and assembly of proteins.
Molecular Formula:
C49H93NO17S
InChI: InChI=1/C37H67NO13.C12H26O4S/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26;1-2-3-4-5-6-7-8-9-10-11-12-16-17(13,14)15/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3;2-12H2,1H3,(H,13,14,15)/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-;/m1./s1/f/h;13H
InChIKey: InChIKey=SKDGGFHGLZBNBC-DKLAHUOIDD
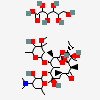
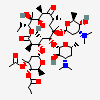
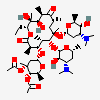
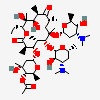
SMILES: CCCCCCCCCCCCOS(O)(=O)=O.CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
Names:
CHEBI:4846
ERYTHROMYCIN ESTOLATE
Erythromycin estolate
erythromycin estolate
Erythromycin estorate
Erythromycin 2'-propionate dodecyl sulfate (salt)
Erythromycin 2'-propionate dodecyl sulfate (salt)
Erythromycin, propionate (ester), compd. with monododecyl
Ilosone (TN)
Lauryl sulfate propionyl erythromycin ester
(3R,4S,5R,6R,7R,9R,11R,12R,13S,14R)-6-[(2S,3R,4S,6R)-4-dimethylamino-3-hydroxy-6-methyl-oxan-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyl-oxan-2-yl]oxy-3,5,7,9,11,13-hexamethyl-1-oxacyclotetradecane-2,10-dione; 1-sulfooxydodecane
3521-62-8
Registries:
PubChem CID 5702270
ChEBI 4846
Kegg C08031
Kegg D00851
PubChem ID 10231
PubChem ID 11533961
|

|
|
|
|
|
|
|
|
|
|
|

|
|
|
|
|
|
|
|
|