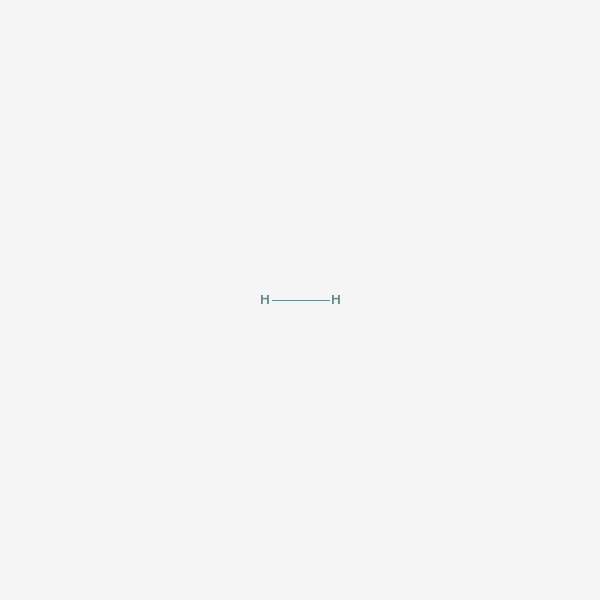
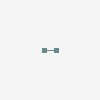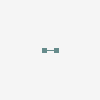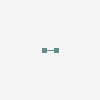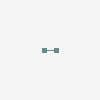molecular hydrogen
 PubChem Notes:
PubChem Notes:
Hydrogen Hydrogen. The first chemical element in the periodic table. It has the atomic symbol H, atomic number 1, and atomic weight 1. It exists, under normal conditions, as a colorless, odorless, tasteless, diatomic gas. Hydrogen ions are PROTONS. Besides the common H1 isotope, hydrogen exists as the stable isotope DEUTERIUM and the unstable, radioactive isotope TRITIUM.
Hydrogen - definition from Biology-Online.org
[(Science: chemistry, element) hydrogen is a gas element which has an atomic number of 1 and an atomic weight of 1.0079. It combines with oxygen to form water (H20) and is present in all organic compounds. a few types of bacteria can metabolise atmospheric hydrogen (H2). hydrogen gas itself is not poisonous, but when it mixes with air it can easily ignite or explode. hydrogen was discovered by henry Cavendish in 1766 and was named by Lavoisier. there are two main isotopes of hydrogen: deuterium (2H) and tritium (3H, which is radioactive and is used in some glow-in-the-dark paints and as a tracer in biological studies). Abbreviation: H
|

|
|

|

|

|

|

|

|

|

|

|
|

|
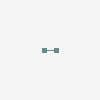
|

|

|

|

|

|

|