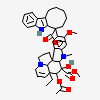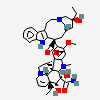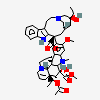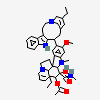vindesine sulfate
 PubChem Notes:
PubChem Notes:
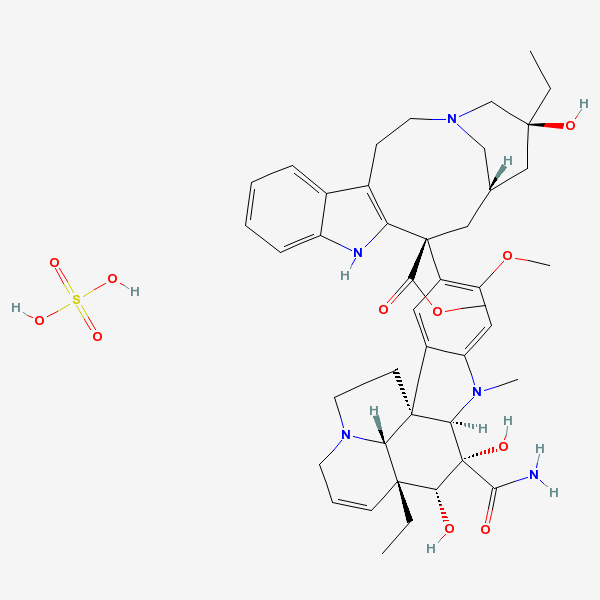
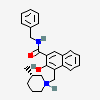
Vindesine Vinblastine derivative with antineoplastic activity against acute leukemia, lung cancer, carcinoma of the breast, squamous cell carcinoma of the esophagus, head, and neck, and Hodgkin's and non-Hodgkin's lymphomas. Major side effects are myelosuppression and neurotoxicity. Vindesine is used extensively in chemotherapy protocols.
Molecular Formula:
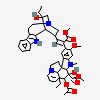
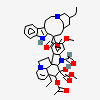
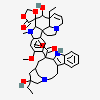
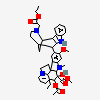
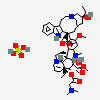
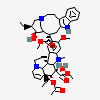
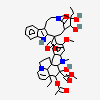
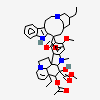
C43H57N5O11S
InChI: InChI=1/C43H55N5O7.H2O4S/c1-6-39(52)21-25-22-42(38(51)55-5,33-27(13-17-47(23-25)24-39)26-11-8-9-12-30(26)45-33)29-19-28-31(20-32(29)54-4)46(3)35-41(28)15-18-48-16-10-14-40(7-2,34(41)48)36(49)43(35,53)37(44)50;1-5(2,3)4/h8-12,14,19-20,25,34-36,45,49,52-53H,6-7,13,15-18,21-24H2,1-5H3,(H2,44,50);(H2,1,2,3,4)/t25-,34+,35-,36-,39+,40-,41-,42+,43+;/m1./s1/f/h44H2;1-2H
InChIKey: InChIKey=COFJBSXICYYSKG-UNOJNIRPDL
SMILES: CCC1(CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C)(C(=O)N)O)O)CC)OC)C(=O)OC)O.OS(=O)(=O)O
Names:
DAVA
Desacetylvinblastine amide sulfate
EINECS 261-984-7
Eldesine
Eldisine
Lilly 99094
Vindesina sulfato [Spanish]
Vindesine sulfate salt
Vindesine sulfate [USAN:JAN]
vindesine sulfate
Registries:
PubChem CID 43116
PubChem ID 183565
|
|
|
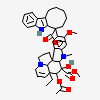
|
|
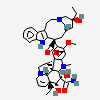
|
|
|
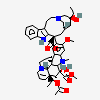
|
|
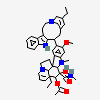
|
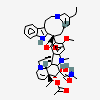
|
|
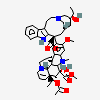
|
|
|
|
|
|
|
|