Ingredients --
Sodium citrate
Chemical Formula:
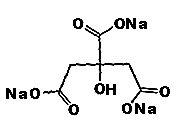
Synonyms
Trisodium citrateDescription
White odorless crystalsUses
Sodium citrate is used in ice cream to keep the fat globules from sticking together. Citrates and phosphates both have this property. It is also an anti-coagulant. As a buffering agent, sodium citrate helps maintain pH levels in soft drinks. As a sequestering agent, sodium citrate attaches to calcium ions in water, keeping them from interfering with detergents and soaps. Compounds with similar functions are sodium carbonate, sodium edta, phosphoric acid, pentasodium pentetate, Tetrasodium etidronate. and Tetrasodium pyrophosphate.sodium citrate: InChI=1/C6H8O7.2Na/c7-3(8)1-6(13,5(11)12)2-4(9)10;;/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12);;/q;2*+1/p-2/fC6H6O7.2Na/h11H;;/q-2;2m
edta: InChI=1/C10H16N2O8/c13-7(14)3-11(4-8(15)16)1-2-12(5-9(17)18)6-10(19)20/h1-6H2,(H,13,14)(H,15,16)(H,17,18)(H,19,20)/f/h13,15,17,19H
sodium carbonate: InChI=1/CH2O3.Na/c2-1(3)4;/h(H2,2,3,4);/q;+1/p-2/fCO3.Na/q-2;m
tetrasodium edta: InChI=1/C10H16N2O8.4Na/c13-7(14)3-11(4-8(15)16)1-2-12(5-9(17)18)6-10(19)20;;;;/h1-6H2,(H,13,14)(H,15,16)(H,17,18)(H,19,20);;;;/q;4*+1/p-4/fC10H12N2O8.4Na/q-4;4m
pentasodium pentetate: InChI=1/C14H23N3O10.5Na/c18-10(19)5-15(1-3-16(6-11(20)21)7-12(22)23)2-4-17(8-13(24)25)9-14(26)27;;;;;/h1-9H2,(H,18,19)(H,20,21)(H,22,23)(H,24,25)(H,26,27);;;;;/q;5*+1/p-5/fC14H18N3O10.5Na/q-5;5m
phosphoric acid: InChI=1/H3O4P/c1-5(2,3)4/h(H3,1,2,3,4)/f/h1-3H
By Simon Quellen Field