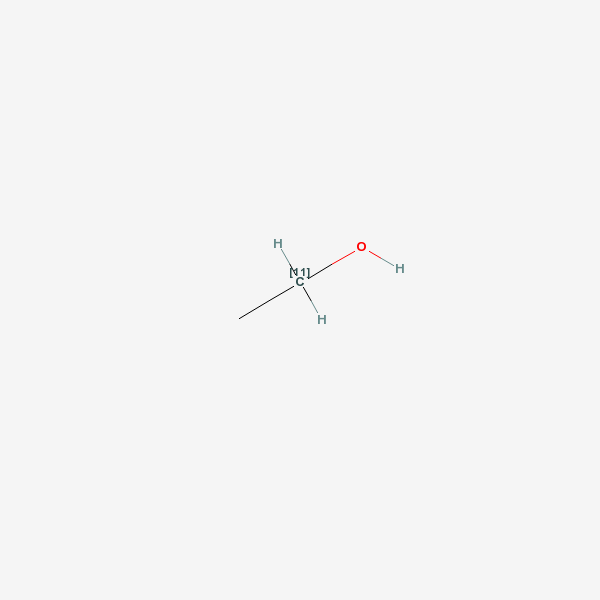
ethanol
 MediLexicon ethanol - Medical Dictionary Definition for Term 'ethanol'
MediLexicon ethanol - Medical Dictionary Definition for Term 'ethanol'
[1. A clear, colorless liquid with a faint ethereal odor and a burning taste, which melts at −114.1°C, boils at 78.5°C, and has a density of 0.789 g/mL at 20°C. It is miscible with water and many organic liquids. Ethanol consumed in beer, wine, and distilled liquor is made by fermentation of sugars obtained from natural sources (grain, grapes, potatoes, sugar cane). Both the aroma of an alcoholic beverage and the smell noted on the breath of a person who has consumed it are due in part to the presence of congeners formed during brewing or fermentation and also due to added flavoring agents. Some alcohol used in industry is synthesized from ethylene or acetylene. Fermentation yields a maximum ethanol concentration of about 14%, above which fermentative enzymes are inhibited or destroyed. Higher alcohol concentrations in beverages are obtained either by addition of pure alcohol (fortified wines) or by distillation (whiskey, gin, vodka). The proof number of an alcoholic beverage represents twice the percent by volume of ethanol (120 proof = 60% ethanol by volume). Concentrations up to 95% can be obtained by fractional distillation. Because the combination of 95% ethanol and 5% water is an azeotropic mixture, incapable of being further concentrated by distillation, higher concentrations are obtained with dehydrating agents. In medicine, ethanol is used topically as a rubefacient, coolant, and disinfectant; internally as an analgesic and sedative; and as a solvent or vehicle for other agents. Alcohol USP contains not less than 92.3% nor more than 93.8% ethanol by weight. An alcoholic solution of a nonvolatile substance (benzoin) is called a tincture. If the solute is volatile (menthol), the solution is called a spirit. Ethanol is widely used in industry as a preservative, solvent, and antifreeze and in the manufacture of perfumes, paints, lacquers, and explosives. It also finds employment as an octane booster in automotive fuels. Most industrial ethanol is denatured by the adition of 1–2% of toxic substances to prevent its use as a beverage.
|

|
|
|
|
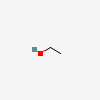
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|