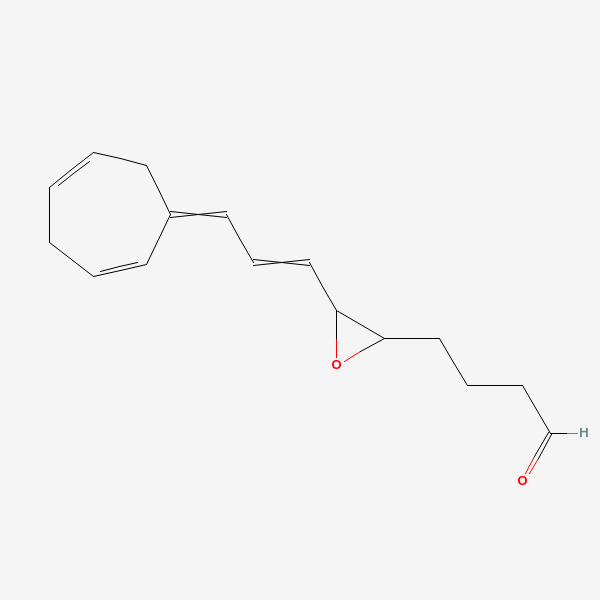
leukotriene-A4
 PubChem Notes:
PubChem Notes:
Leukotriene A4 (2S-(2 alpha,3 beta(1E,3E,5Z,8Z)))-3-(1,3,5,8-Tetradecatetraenyl)oxiranebutanoic acid. An unstable allylic epoxide, formed from the immediate precursor 5-HPETE via the stereospecific removal of a proton at C-10 and dehydration. Its biological actions are determined primarily by its metabolites, i.e., LEUKOTRIENE B4 and cysteinyl-leukotrienes. Alternatively, leukotriene A4 is converted into LEUKOTRIENE C4 by glutathione-S-transferase or into 5,6-di-HETE by the epoxide-hydrolase. (From Dictionary of Prostaglandins and Related Compounds, 1990)
Molecular Formula:
C16H20O2
InChI: InChI=1/C16H20O2/c17-13-6-5-11-15-16(18-15)12-7-10-14-8-3-1-2-4-9-14/h1,3-4,7,9-10,12-13,15-16H,2,5-6,8,11H2
InChIKey: InChIKey=HWUPLFSLSVKTJN-UHFFFAOYAW
SMILES: C1C=CCC(=CC=CC2C(O2)CCCC=O)C=C1
Names:
leukotriene-A4
LTA4
(7E, 9E, 11Z, 14Z)-(5S, 6S)-5, 6-Epoxyeicosa-7, 9,11, 14-tetraenoate
(7E, 9E, 11Z, 14Z)-(5S, 6S)-5, 6-Epoxyicosa-7, 9,11, 14-tetraenoate
4-[3-[3-(1-cyclohepta-2,5-dienylidene)prop-1-enyl]oxiran-2-yl]butanal
72059-45-1
Registries:
PubChem CID 858
PubChem ID 2907
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|