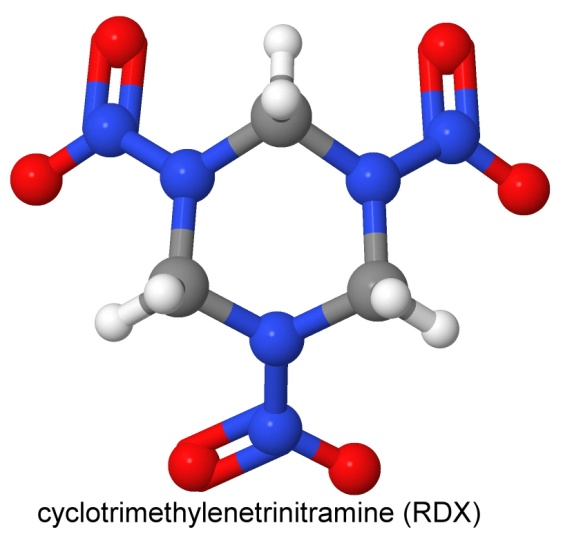
The name RDX stands for Research Department eXplosive. This was a code name for the explosive known in the U.K as Cyclonite, in Germany and Russia as hexogen, and in Italy as T4.
RDX was patented in 1898 by the German chemist Georg Friederich Henning. He prepared it by nitrating hexamine nitrate. His original patent (German patent 104280) mentioned medical uses, but subsequent patents suggested its use in smokeless propellants.
The original patent is short enough to show here in its entirety:
Patent issued in the German Republic on July 15, 1898.
Dr. G.F. HENNING, Berlin, Germany
Process for Making a Nitro compound from Hexamethylenetetramine
Hexamethylenetetramine cannot be nitrated using the commonly known nitration methods; the tetramine compound decomposes into ammonia and formaldehyde. It is, however, possible, using a bypass to obtain a nitro compound, which has remarkable properties. Dissolving hexamethylenetetramine in 3 parts of water and cooled to 0° C, adding this solution to cooled concentrated nitric acid, very soon the crystallization of nitric acidic hexamethylenetetramine begins. This salt must be washed with ice water and cold ethanol and dried immediately. Otherwise, it decomposes forming formaldehyde.
For making the nitro compound 10 parts of the thoroughly dried salt is added in small portions to 50 parts concentrated nitric acid which is cooled to -5°C and has a specific weight of 1.52, after each addition waiting for ceasing the mild foaming caused by the reaction, before added a new portion. After adding the entire amount of the nitrate, the beaker containing the nitrate is allowed to stand in the cooling mixture 30 minutes and pouring the acid in a small stream in ice water. Immediately the precipitation of the colorless crystals begins which are dried in air after it was thoroughly washed with water.
The yield is such that the amount of nitro compound made as the amount of hexamethylenetetramine was used. The previously unknown compound melts at 200°C under decomposition; its composition is as follows:
38.34 % nitrogen
16.45 % carbon
2.81 % hydrogen
42.40 % oxygen
The simplest net formula is C3N6H6O6.
The compound does not dissolve in water; it dissolves slowly in boiling alcohol, however, it dissolves easily in acetone in which it can be made in any crystal sizes. The solution in acetone reacts acidic. The compound is also easily dissoluble in concentrated acetic acid and precipitates forming crystals when diluted with water. The nitro compound does not dissolve in diluted acids, as well as in diluted alkali. If heated in potassium or sodium hydroxide it decomposes, and the decomposition continues without any further heating once it is initiated. Among the decomposition products was nitrogen and oxygen. When rapidly heated the compound explodes with a bang without any residue; it is not sensitive against blow and stroke, its storage stability is unlimited. The novel nitro compound does not have an odor and neither taste, however, it forms easily formaldehyde in the presence of reduction and oxidation agents; the nitro compound has technical applications and serves as starting material for medicinal products.
Claim:
A process for making a nitro compound from hexamethylenetetramine by adding nitric acidic hexamethylenetetramine in cold concentrated nitric acid.
In 1920, Austrian (later a citizen of Germany) Edmund von Herz filed a patent (US1402693) on an improved method for making it.
During World War II, several new methods for making RDX were developed, several in Germany, and more each in the U.S., Canada, and the U.K.
Specifically, the American chemist Werner E. Bachmann and his newly minted Ph.D. student John Clark Sheehan developed a method involving the nitration of hexamethylenetetramine.
Sheehan recalls wearing the standard lab coat and safety glasses, but also a heavy towel wrapped around his neck to protect it from flying glass. They made the explosive at a kilogram scale, and the process was later scaled up further by the company Tennessee Eastman (part of Eastman Kodak) for the war effort. Because the Bachmann process was a continuous flow method, not the batch methods in use elsewhere, it could keep up with the war demands more easily.
During the war, Britain was finding it difficult to destroy German U-boats by air, using only TNT-based explosives. RDX, being much more powerful, was used in a mixture containing TNT and aluminum powder, resulting in half-again more powerful depth charges that could be dropped by aircraft after sighting an enemy submarine. The mixture, called Torpex, was also used to burst dams, in a type of bomb that would skip over the water to avoid torpedo nets before hitting the dam and submerging to detonate far underwater and produce the highest shockwave.
In the U.S., wartime production of RDX amounted to 15 thousand tons a month. In Germany, another 7 thousand tons a month were produced. While more expensive to manufacture than TNT, RDX had twice the explosive power as the same volume of TNT.
RDX has a very high detonation velocity — the densest form detonates at 8750 meters per second.
RDX became a mainstay in military explosives, almost as much as TNT had been before it. It was mixed in a variety of forms under a number of names:
- Torpex — 42% RDX, 40%TNT, 18% powdered aluminum
- Semtex — 50% RDX, 50% PETN
- Cyclotol — A castable explosive mixture of TNT and RDX in various proportions
- Composition A — RDX and wax, used to desensitize it and make a moldable explosive
- Composition B — 60% RDX and 40% TNT, plus some desensitizing wax
- Composition C — 91% RDX mixed with a plasticizer, a binder, and motor oil
- PBX — Polymer-bonded explosive, a huge number of plastic explosive formulas
Composition C was later sub-divided into several types, C-2, C-3, and C-4.
The starting point in the synthesis of RDX is methyl alcohol. As we saw with tetryl, methyl alcohol (produced by the distillation of wood) was a scarce commodity during the First World War, limiting the availability of both tetryl and RDX.
In 1921, the French chemist Georges Patart patented a method of producing methyl alcohol from carbon monoxide and hydrogen, using catalysts and high pressure. Improved catalysts developed in Germany allowed the industrial production of large quantities of methyl alcohol, thereby destroying the wood distillation industry.
By the late 1920's, methyl alcohol was being produced in the U.S. by yet newer methods, starting with coal as the base for both the carbon monoxide and hydrogen, made by passing steam over hot coke (a by-product of the distillation of coal).
By adding extra hydrogen (from reacting steam with hot iron), methyl alcohol could be made from carbon dioxide instead of carbon monoxide, and since large amounts of carbon dioxide were available from industrial fermentation plants, the price of the alcohol went down, and the availability was limitless.
Reacting methyl alcohol with a hot copper screen as a catalyst produces formaldehyde, a starting point for the production of RDX.
After 1926, RDX could be made from nothing other than coal, water, and air. With virtually unlimited access to the starting materials, only the lengthy synthesis process made tetryl and RDX more expensive than the less powerful TNT.